Phytosterol esterification product and method of make same
a technology of esterification product and phytosterol, which is applied in the field of phytosterols, can solve the problems of brittle product, difficult to incorporate free sterols into edible fats or oils, and pose challenges in the manufacture of food products for consumer use, and achieve the effect of greater cholesterol-lowering potential
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0040] A mixture of 60% phytopowder, 38% palm flakes and 2% glycerin was charged to a reaction flask with a nitrogen sparge, an agitator, and a stainless steel temperature probe. This same reaction flask with nitrogen sparge, agitator, and temperature probe was used in each of the following examples. The mixture was brought to a temperature of about 160° C., and stannous chloride catalyst was added. The temperature was brought to about 180-210° C., and the esterification reaction was allowed to proceed for about 2.5 hours under vacuum. Difficulties were noted with effective removal of evolved steam during the esterification reaction. The reaction mixture then was treated with 0.7% citric acid neutralizing agent for about 10 minutes. The neutralized material was treated with Filter Aid and Bleaching clay for about 10 minutes, and then filtered. It was determined that the esterification reaction was 44.6% complete. The final product had 8.35 ppm tin as residual catalyst, and 0.3% mois...
example 2
[0041] A mixture of 75% phytopowder, 20% palm flakes and 5% glycerin was charged to the reaction flask. The mixture was brought to a temperature of about 160° C., and stannous chloride catalyst was added. The method was carried out as described above, except that the temperature of the esterification reaction was in the range of about 180-230° C., and steam was more effectively removed by use of two cold fingers. It was determined that the esterification reaction was 48.3% complete. The final product had 34.90 ppm tin as residual catalyst.
example 3
[0042] A mixture of 60% phytopowder, 20% palm flakes, 15% high oleic canola oil and 5% glycerin was charged to the reaction flask. The mixture was brought to a temperature of about 160° C., and stannous chloride catalyst was added. The method was carried out as described above, except that the temperature of the esterification reaction was in the range of about 180-225° C., and the neutralization reaction time was increased to about 20 minutes. It was determined that the esterification reaction was only 6.9% complete. The final product had 1.01 ppm tin as residual catalyst.
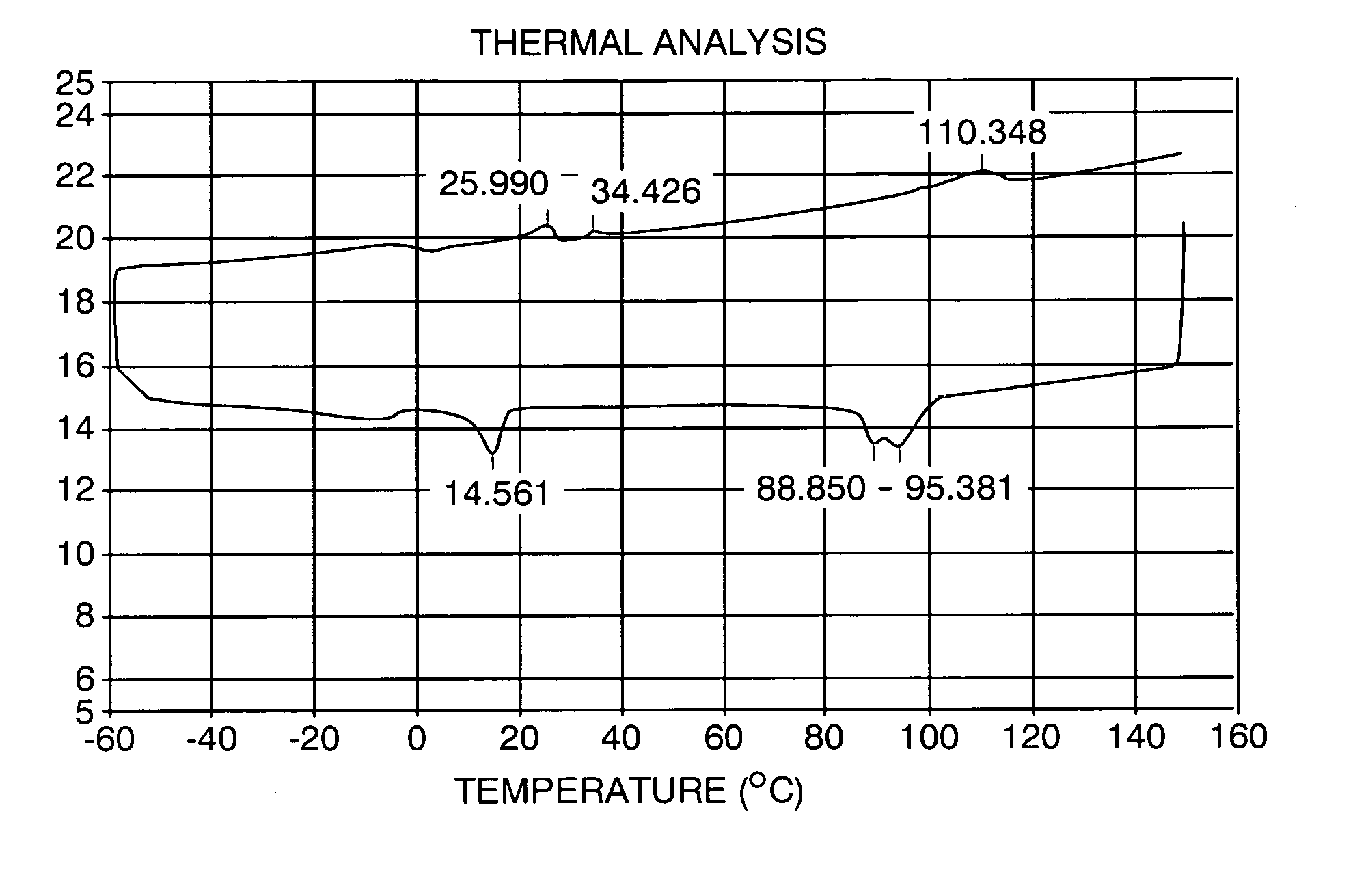
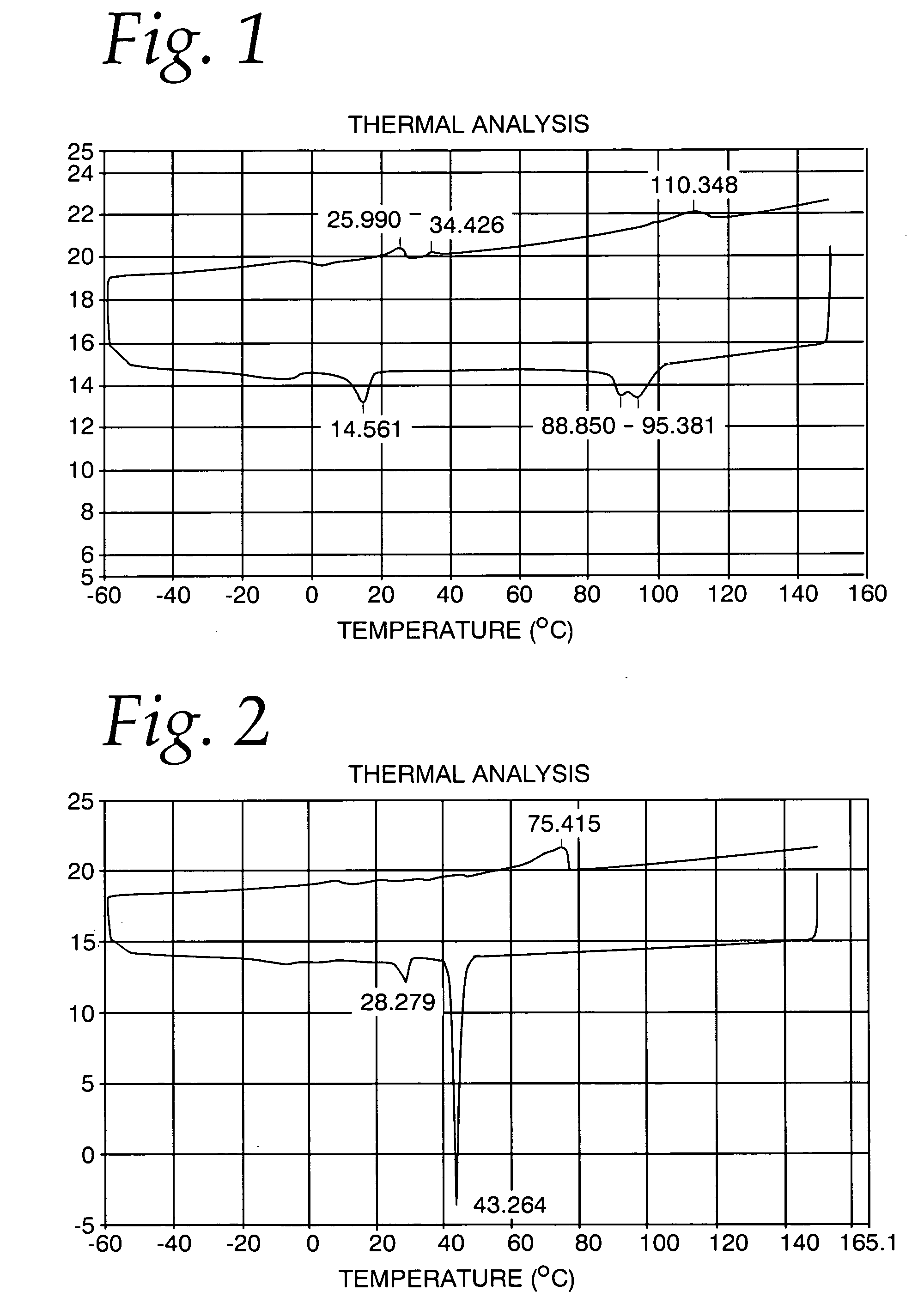
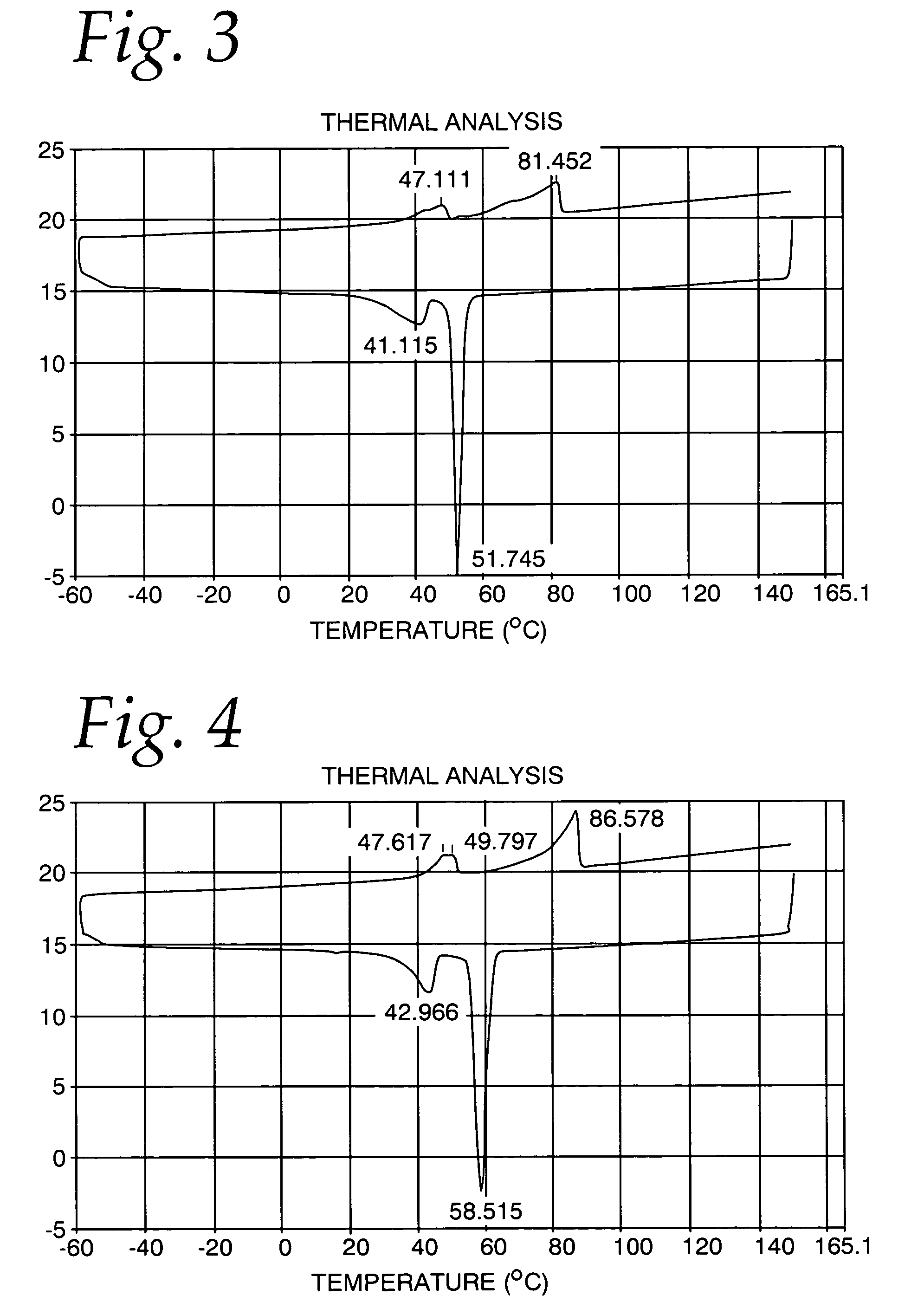
[0043] A differential scanning calorimetry analysis for this product is shown in FIG. 1. It may be seen that the melting curve show a first peak at about 26 C, followed by a second peak at about 34 C, and a final peak at about 110 C. The cooling curve shows a first peak at about 95 C, a second peak at about 89 C, followed by a third peak at abut 14.5 C. It may be seen that the peaks are relatively broad, indicati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com