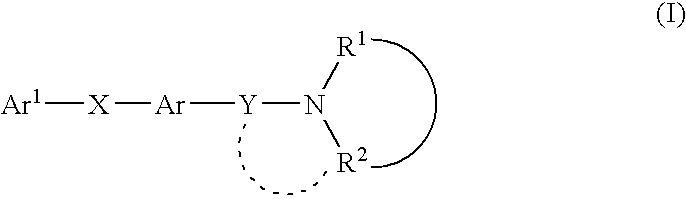
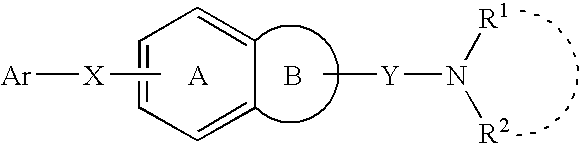
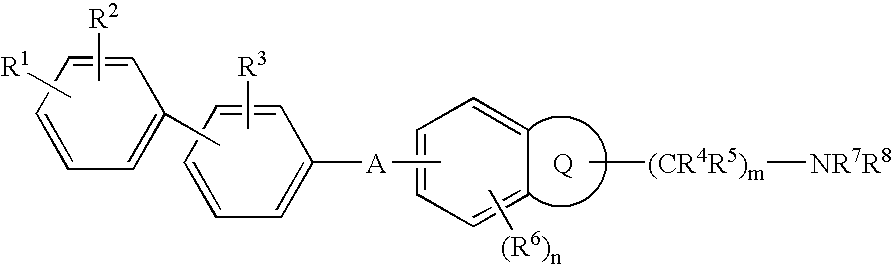
Melanin concentrating hormone antagonist
a technology of concentrating hormone and antagonistic action, which is applied in the field of concentrating hormone antagonists, can solve the problems of arthritis and pain, obesity is now becoming a social problem, and achieve the effect of excellent mch antagonistic action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
reference example 1
2-(R)-[2-(N,N-Dimethylamino)ethy]-6-(4-[(4-methoxyphenyl)carbonyloxy]benzyloxy)tetralin
Diethyl azodicarboxylate (40% toluene solution, 0.95 g) was added dropwise to THF solution (6 ml) of 2-(R)-[2-(N,N-dimethylamino)ethyl]-6-hydroxytetralin (300 mg), 4-(hydroxymethyl)phenyl 4-methoxybenzoate (530 mg), and triphenylphosphine (430 mg) under ice-cooling. After stirring for 2 hours at room temperature, the reaction mixture was concentrated. The residue was purified using alumina column chromatography (development solvent; hexane˜hexane: ethyl acetate=10:1), and the titled compound (320 mg) was obtained after recrystallization (ethyl acetate-hexane).
Melting point: 111-114° C.
[α]D20=+44.4° (c=0.502, methanol)
reference example 2
N-Phenyl-4-[[2-(2-piperidinoethyl)-6-tetralinyl]oxymethyl]benzamide
Triethylamine (0.11 ml) was added to THF suspension (3 ml) of 4-[[2-(2-piperidinoethyl)-6-tetralinyl]oxymethyl]benzoate (300 mg). Further, THF solution (0.5 ml) of trimethylacetyl chloride (92 mg) was added dropwise under ice-cooling, which was stirred for 30 minutes. The temperature of the reaction mixture was raised to room temperature, which was stirred for 1 hour. THF solution (0.5 ml) of aniline (85 mg) was added dropwise to the reaction mixture under ice-cooling, which was stirred for 1 hour. After the reaction mixture was stirred for 24 hours at room temperature, saturated sodium bicarbonate solution was added, and extraction was conducted using a mixed solution of ethyl acetate and THF. The organic layer was washed with water and saturated aqueous sodium chloride solution, dried, and then concentrated. The residue was recrystallized from THF-methanol-IPE to give the titled compound (150 mg).
Melting point:...
references example 3
4-[[2-(2-Piperidinoethyl)-6-tetralinyl]oxymethyl]-N-(2-pyridinyl)benzamide
Triethylamine (0.11 ml) was added to THF suspension (6 ml) of 4-[[2-(2-piperidinoethyl)-6-tetralinyl]oxymethyl]benzoate (300 mg). Trimethylacetyl chloride (0.095 ml) was added dropwise to the obtained suspension under ice-cooling, which was stirred for 30 minutes. The temperature of the reaction mixture was raised to room temperature, which was stirred for 1 hour. THF solution (1.0 ml) of 2-aminopyridine (110 mg) was added dropwise to the reaction mixture under ice-cooling, which was stirred for 1 hour. Then the reaction mixture was stirred at room temperature for 6 hours, and at 60° C. for 12 hours, which was refluxed with heating for 6 hours. Saturated sodium bicarbonate solution was added to the reaction mixture, and extraction was conducted using ethyl acetate. The organic layer was washed with water and saturated aqueous sodium chloride solution, dried, and then concentrated. The residue was purified us...
PUM
| Property | Measurement | Unit |
|---|---|---|
| body weight | aaaaa | aaaaa |
| stress | aaaaa | aaaaa |
| body weights | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com