Apparatus and Method for Monitoring Tissue Vitality Parameters
a technology of vitality and apparatus, applied in the field of apparatus and method for monitoring tissue vitality parameters, can solve the problems of limiting the availability of this process, reducing the form of the molecule, and unable to meet the demand in the tissue or cell, so as to avoid photo-bleaching of the measured, monitor the nadh level, and correct efficiently for the haemodynamic artifact
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
first embodiment
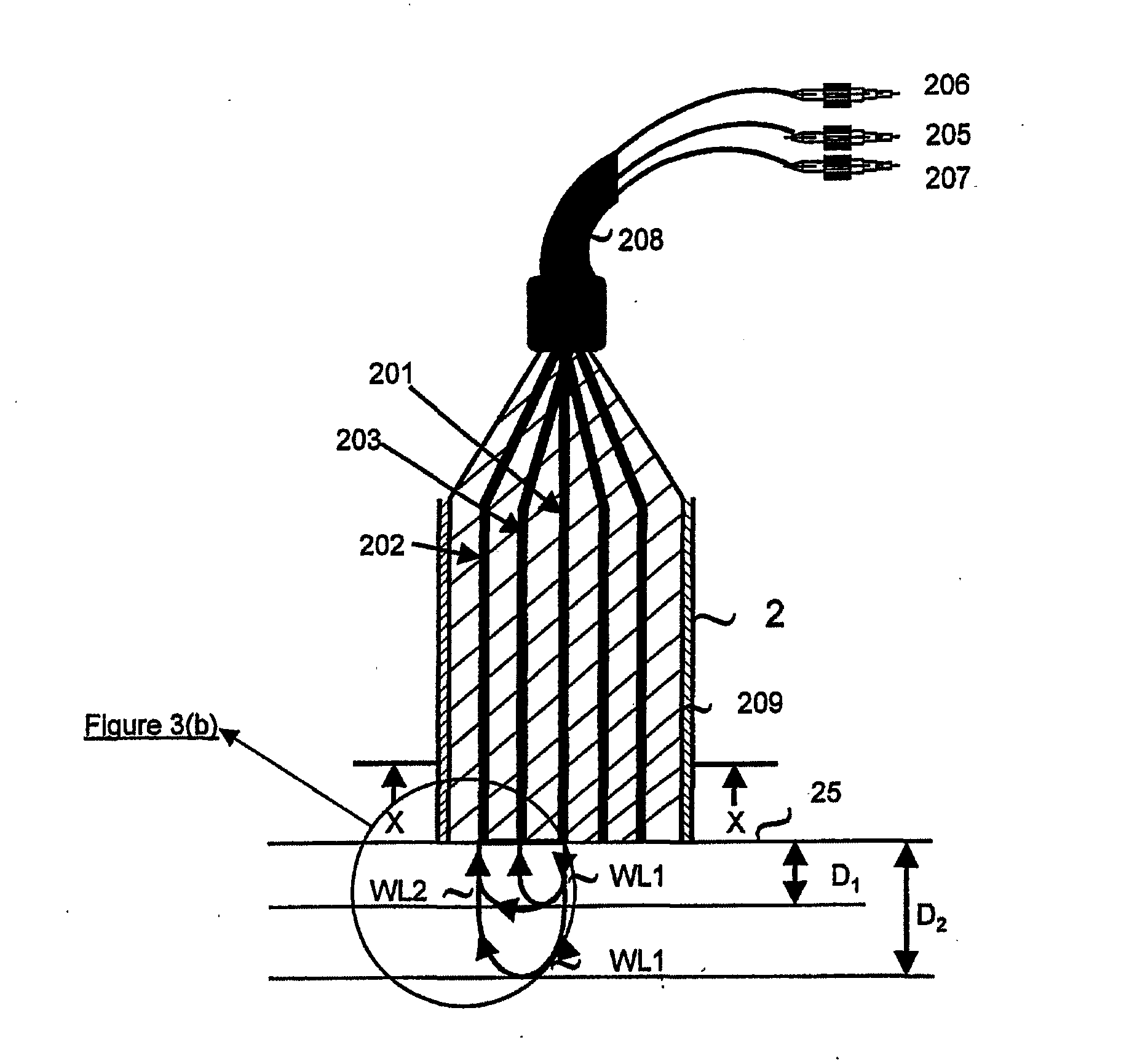
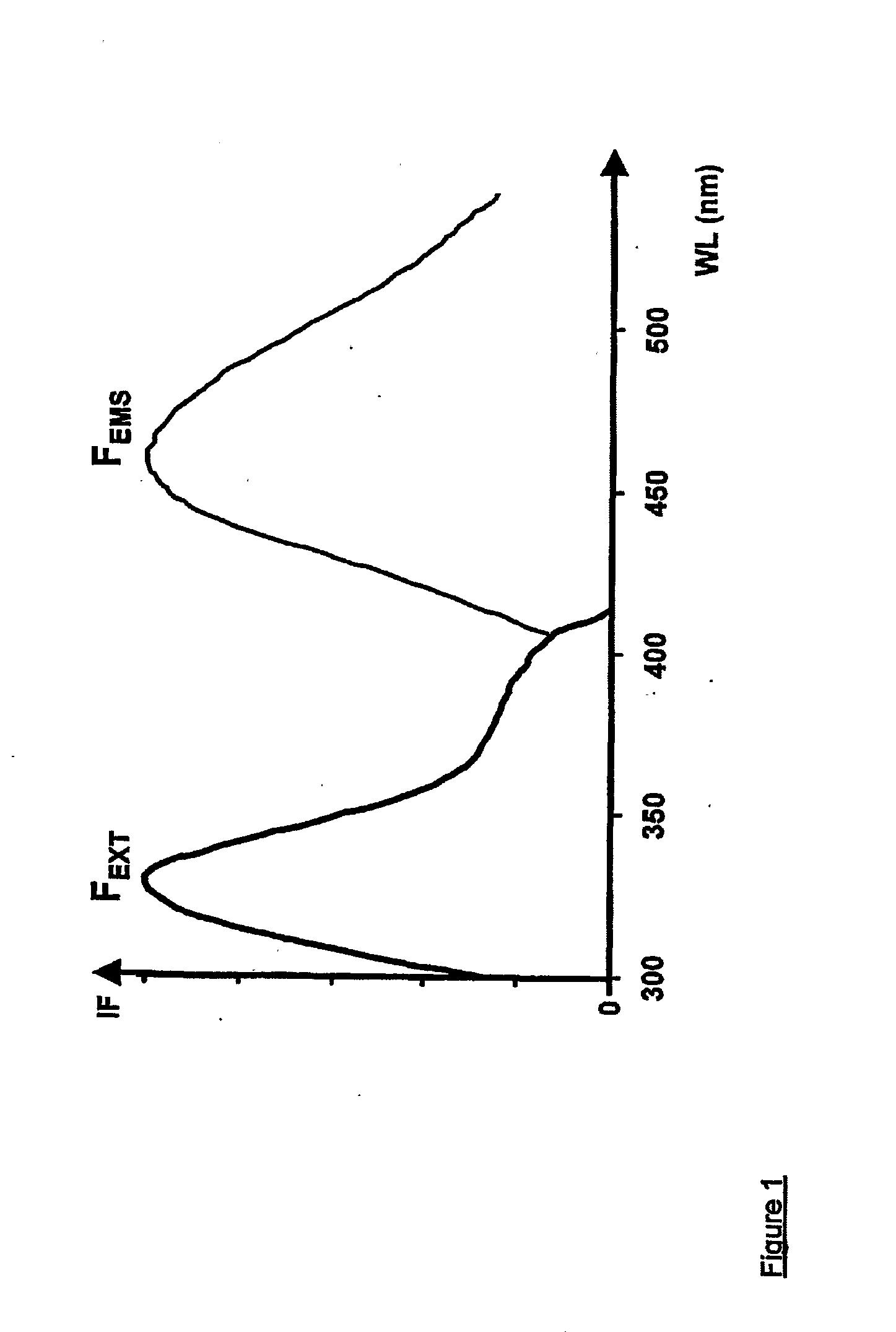
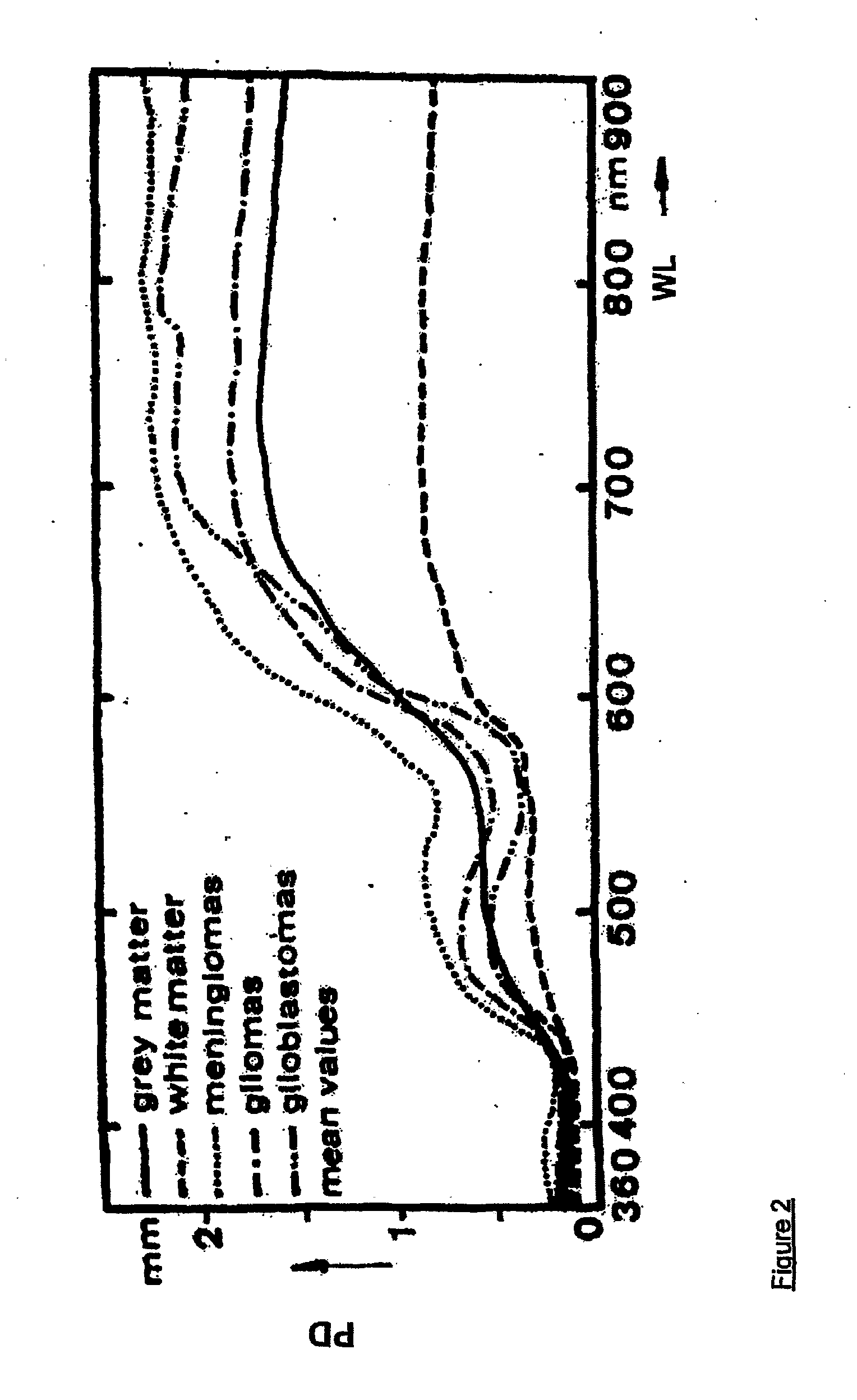
[0145] Thus, in the first aspect of the present invention, and referring to FIGS. 3(a), 3(b), 4(a) and 5, the apparatus generally designated by the numeral (100), is directed to the monitoring of blood flow rate of the first set of tissue viability parameters, and any combination of the three tissue viability parameters of the second set of tissue viability parameters. Thus, the apparatus (100) may be in the form of a probe (2) having at the distal tip thereof contact face (12) for making contact with the surface of the tissue (25) being monitored. In its simplest form, the probe (2) has a single fiber (201) for directing two radiations of different wavelengths to the same point (15) on the tissue (25). Alternatively, a bundle of fibers may replace a single fiber (201). The two radiations may come from first and second sources, (22) and (24) respectively, and are coupled to the fiber (201) by any suitable optical coupler. Referring to FIG. 2, other than at the plateau of wavelength...
second embodiment
[0194] In embodiments where the Doppler excitation wavelength WL1 lies in the range 420 nm to 440 nm, in either the first or second embodiment, the resulting NADH fluorescence will not pass through the dichroic beam splitter (302) designed to split these wavelengths towards detector (301). To overcome this problem at these wavelengths, a simple 1:20 beam-splitter should be substituted for the fore-mentioned dichroic beam splitter. It should be noted, that the strong WL1 reflection will not interfere with the NADH fluorescence, despite the two signals having similar wavelengths, because the signal are separated in time.
[0195] While the appropriate illumination wavelengths for sources (101), (102) referred to hereinbefore are particularly suited for the monitoring of brain tissue in-vivo using the first and second embodiments, corresponding illumination wavelengths may be determined for any other tissue enabling the apparatus (100) to be used with such tissues, mutatis mutandis.
[0196...
fifth embodiment
[0266] The fifth embodiment may be operated in a variety of modes as required by the clinical situation and diagnostic needs to which it is applied. Two particular modes of monitoring for which such multiple probe systems can be usefully applied, are described:
[0267] In the first mode, the mean signal intensities from the multiplicity of probes is calculated and displayed. This results in the parameters detected representing an average response of the multiplicity of tissue volumes probed, and will generally, better reflect the state of the organ layer as a whole. This mode of monitoring could be useful in transplantation surgery when better monitoring of the viability of donated organs are needed.
[0268] In the second mode, by applying one or several of the plurality of probes to each of several locations on the same organ or several different locations of different organs, the quasi-continuous monitoring of these organs over the same time period can be achieved by multiplexing the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com