Polysaccharide compositions for use in tissue augmentation
a polysaccharide composition and tissue technology, applied in the field of tissue augmentation, can solve the problems of reducing affecting the implantation rate of the graft,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of 2.3% Sodium CMC Gel in Sterile Water.
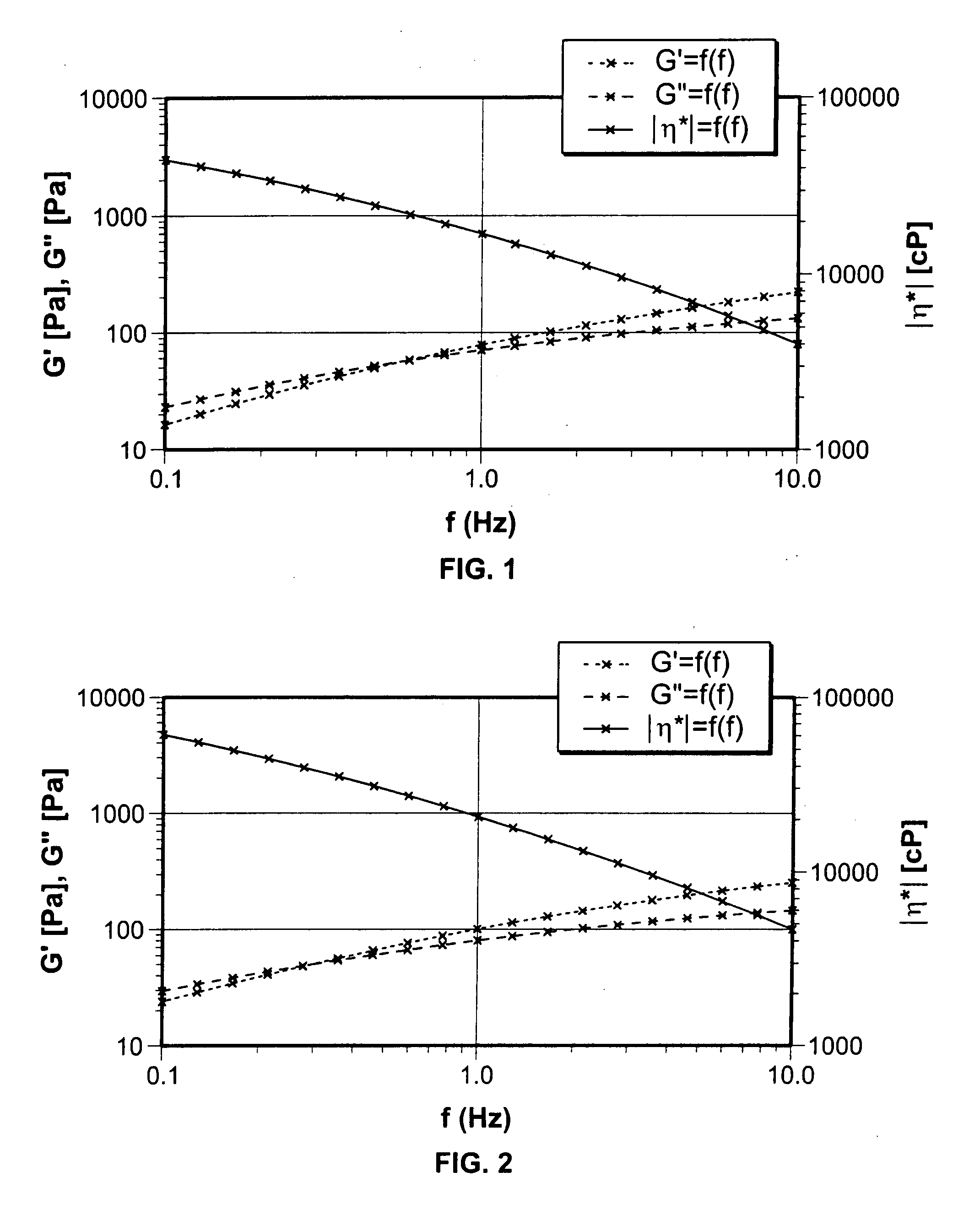
[0087] Sodium carboxymethylcellulose was prepared in sterile water for injection and adjusted to a pH of from about 7.1 to about 8.0 using potassium hydroxide. The dispersion was mixed in an orbital Ross mixer @1725 RPM for 5 minutes followed by mixing in an orbital Ross mixer @1725 RPM for 40 minutes. while holding a vacuum @ 26 mm Hg or more. The composition was then steam sterilization at 121 degrees C. for times ranging from 3 minutes to 30 minutes. In addition, one sample was sterilized for time intervals between 3 minutes and 30 minutes @ 121 degree C. Results are shown in FIG. 1 where G′ represents the elastic modulus, G″ represents the viscous modulus and η the complex viscosity. The profile shows that G′ and G″ intersect at 0.495 Hz (3.2 Rad / sec). Above this frequency, the composition exhibits non-newtonian solution characteristics (tan δ<1.0).
example 2
Preparation of 2.4% Sodium CMC Gel in Sterile Water.
[0088] Sodium carboxymethylcellulose was prepared in sterile water for injection and adjusted to a pH of from about 7.1 to about 8.0 using potassium hydroxide. The dispersion was mixed in an orbital Ross mixer @1725 RPM for 5 minutes followed by mixing in an orbital Ross mixer @1725 RPM for 40 minutes. while holding a vacuum @ 26 mm Hg or more. The composition was then steam sterilization at 121 degrees C. for times ranging from 3 minutes to 30 minutes. In addition, one sample was sterilized for time intervals between 3 minutes and 30 minutes @ 121 degree C. Results are shown in FIG. 2 where G′ represents the elastic modulus, G″ represents the viscous modulus and η the complex viscosity. The profile shows that G′ and G″ intersect at 0.0299 Hz (1.8 Rad / sec) (lower frequency than that shown in FIG. 1). Above this frequency, the composition exhibits non-Newtonian solution characteristics (tan δ<1.0).
example 3
Preparation of 2.5% Sodium CMC Gel in Sterile Water.
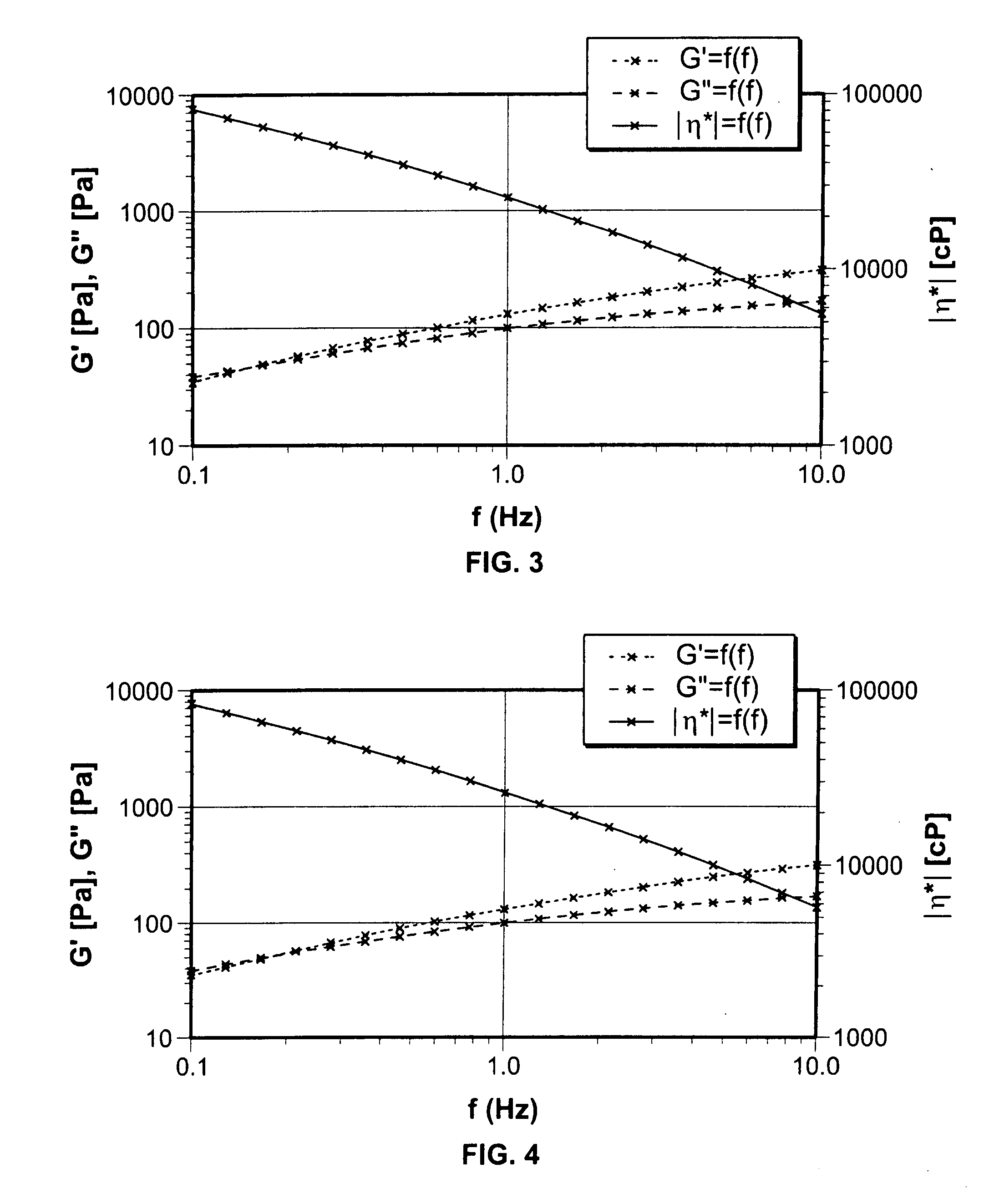
[0089] Sodium carboxymethylcellulose was prepared in sterile water for injection and adjusted to a pH of from about 7.1 to about 8.0 using potassium hydroxide. The dispersion was mixed in an orbital Ross mixer @1725 RPM for 5 minutes followed by mixing in an orbital Ross mixer @1725 RPM for 40 minutes. while holding a vacuum @ 26 mm Hg or more. The composition was then steam sterilization at 121 degrees C. for times ranging from 12 minutes to 30 minutes. In addition, one sample was sterilized for time intervals between 3 minutes and 12 minutes @121 degree C. Results are shown in FIG. 3 where G′ represents the elastic modulus, G″ represents the viscous modulus and η the complex viscosity. The profile shows that G′ and G″ intersect at 0.157 Hz (1 rad / sec) frequency than shown in FIGS. 1 and 2. Above this frequency, the composition exhibits non-Newtonian solution characteristics (tan δ<1.0).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com