Method for providing an active agent topically to the skin
a technology of active agents and skin, applied in the direction of aerosol delivery, inorganic non-active ingredients, extracellular fluid disorder, etc., can solve the problem that the transdermal drug delivery system has not been integrated into the application of topical dressings, and achieve the effect of accelerating wound healing and enhancing the release of active agents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0065] A first experiment was conducted to evaluate the sustained release of protease from a silicone matrix. A loosely or lightly cross-linked silicone elastomer composition (Dow Corning® 9040) and a silicone-based surfactant (Dow Corning® 9011), both commercially available from Dow Corning Corporation (Midland, Mich.), were used to form a Dow Corning® 9040 and a Dow Corning® 9040 / 9011 silicone elastomer formulation. A 1.1 mg / ml protease A, derived from =i B. lentus=l , stock solution dissolved in propylene glycol was added to both Dow Corning® compositions. A 5 ml. sample of the stock solution was added to 20 grams of the 9040 formulation and also to 20 grams of the 9040 / 9011 formulation, which comprises 10 grams of the 9040 formulation and 10 grams of the 9011 formulation. Controls comprising 9040 and 9040 / 9011 plus water instead of the stock enzyme solution were prepared. In addition, to determine whether any component of the silicone matrix was inhibiting the protease, further ...
example 2
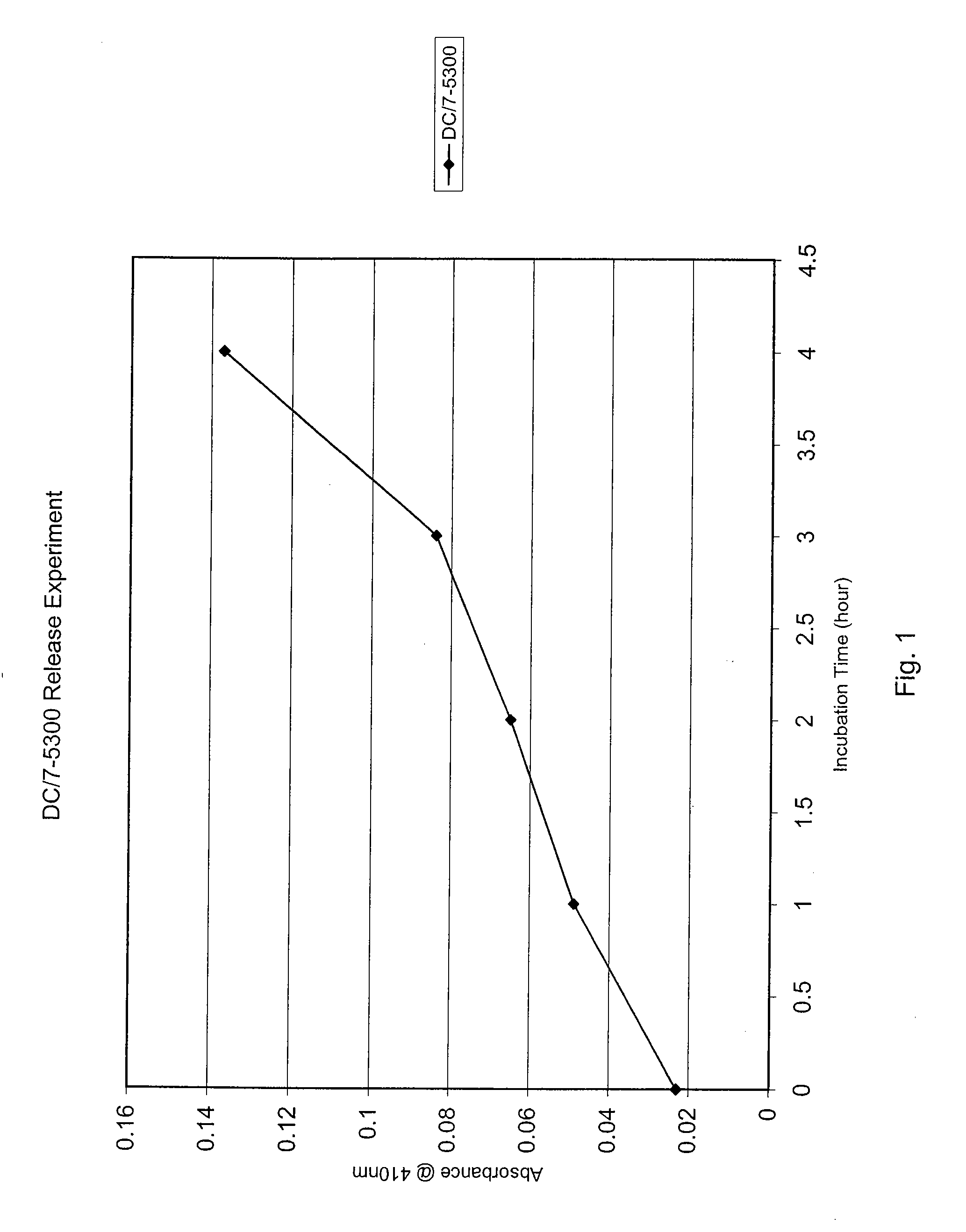
[0067] Another experiment was conducted to evaluate the sustained release of protease from a silicone matrix. A 0.5 ml aliquot of 0.81 mg / ml Protease A in polyethylene glycol stock solution was transferred into a small polypropylene weighing boat. Next, 5.0 ml of a silicone rubber composition (Dow Corning® 7-5300 from Dow Corning Corporation, Midland, Mich.) was added to the protease solution and mixed within 15 seconds of its addition. It is contemplated that the Dow Corning® 7-5300 composition has applications as a “spread-on” film, patch, or bandage. The mixture was then allowed to cure for 30 minutes. Following curing, the mixture was washed three times using 1.0 ml of distilled water. Each wash was assayed using the SAAPFpNA assay on the aliquots, as referenced above, and the amount of enzyme in the wash was measured. The composition was then dried on its side for 15 minutes, followed by an additional 15 minutes laying flat. Finally, 5.0 ml of distilled water was added to the w...
example 3
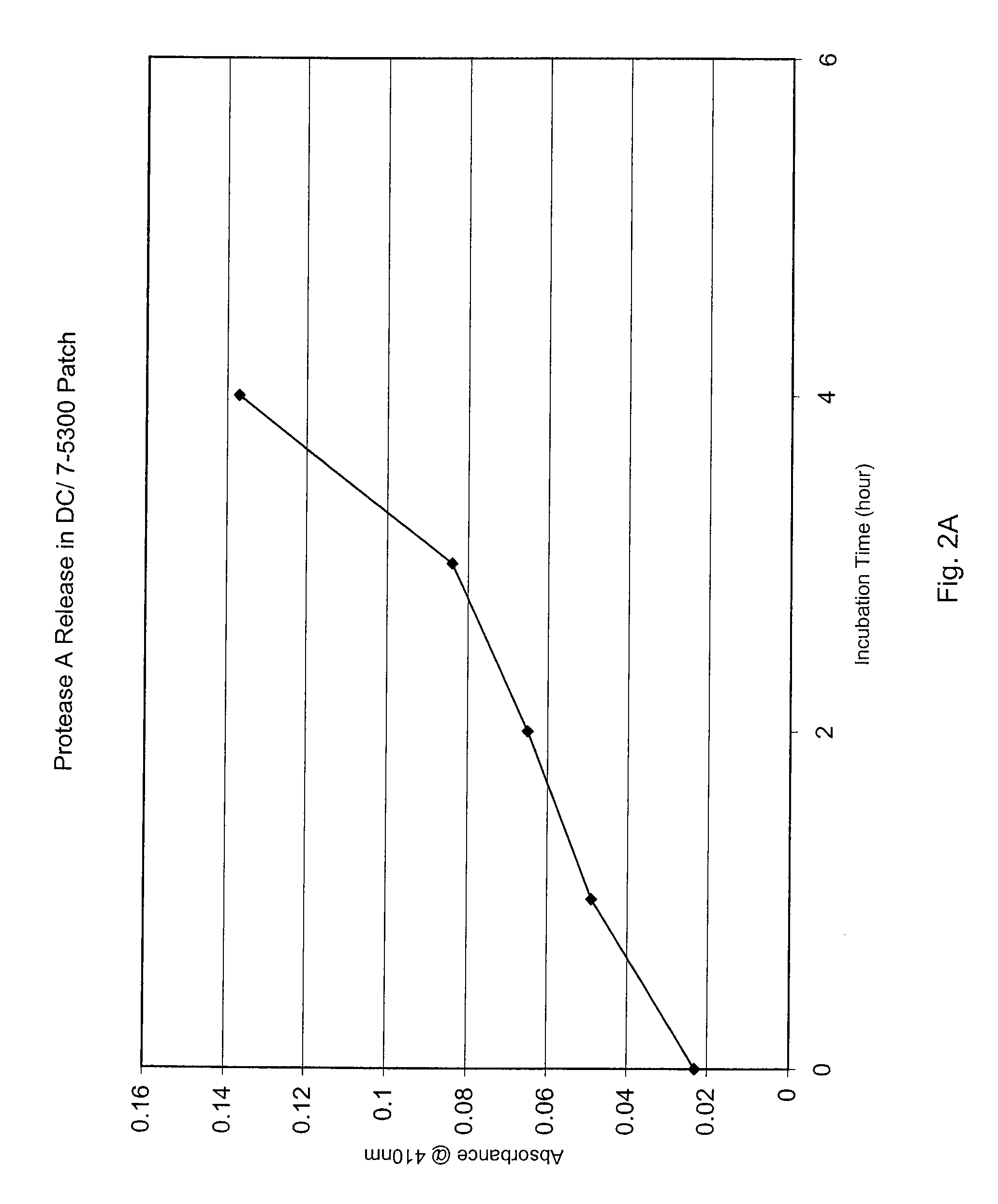
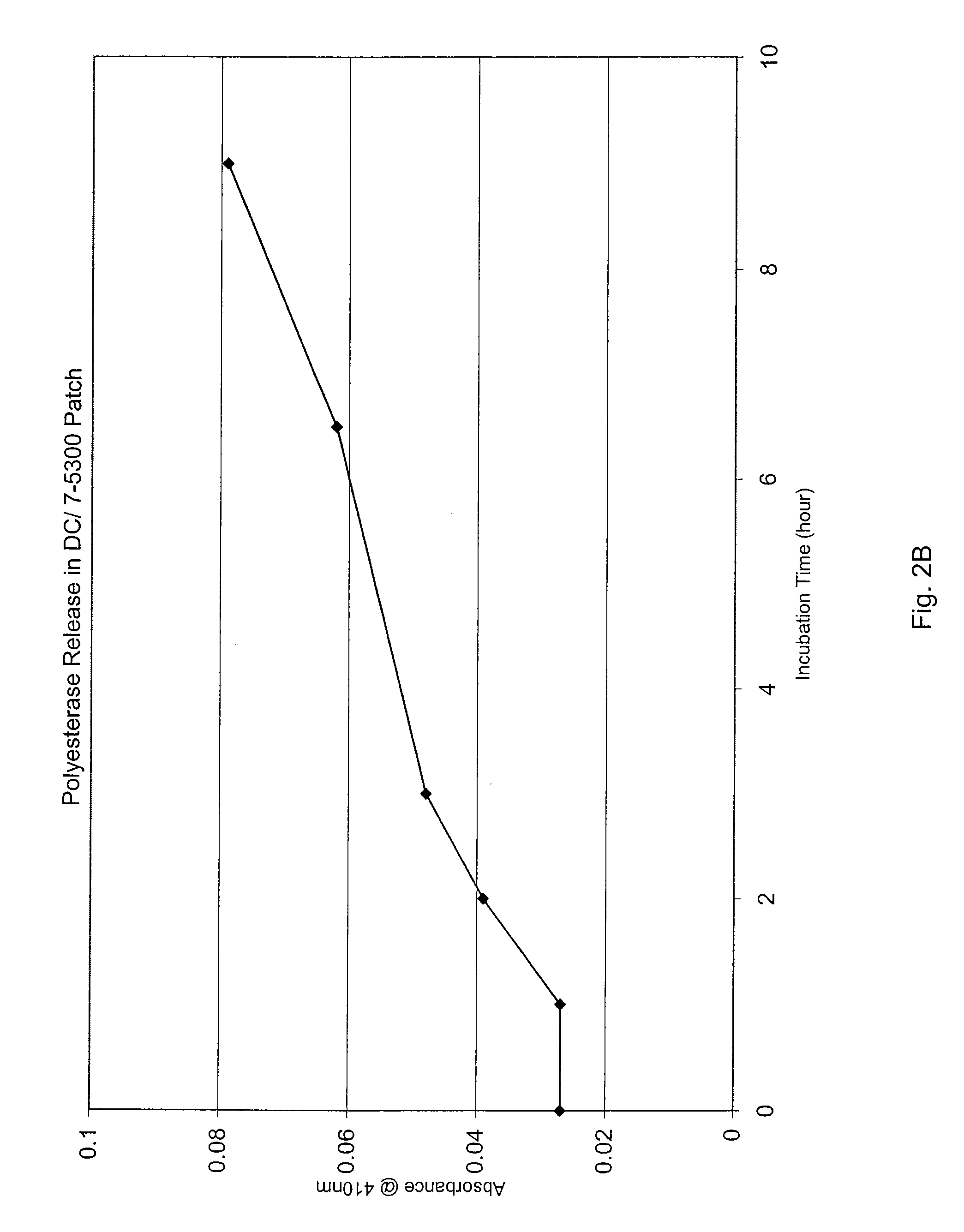
[0071] Still another experiment was conducted to evaluate the effect of hydrophilic additives on the sustained release of Protease A from a silicone matrix. First, test dressings or, more specifically, patches containing protease were cast into small petri-dishes (approximately 3 cm in diameter) such that the total weight of the patches was constant (about 2 grams) and the concentration of enzyme in the patches was also constant (about 0.6 mg agent per gram of patch). The patches were comprised of a loosely or lightly cross-linked silicone elastomer composition (Dow Corning® 9040) and a silicone-based surfactant (Dow Corning® 9011), both commercially available from Dow Corning Corporation (Midland, Mich.). In addition, Dow Corning® 7-5300 (a silicone rubber composition) was also tested. Additionally, the formulations contained varying amounts of PVA, PVA at high propylene glycol levels, or PVP that were added by stirring.
[0072] Enzyme release was evaluated using two methods. In the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com