Mucoadhesive thermoresponsive medicament-carrier composition
a thermoresponsive, medicament-carrier technology, applied in the direction of fluorescence/phosphorescence, inorganic non-active ingredients, peptide/protein ingredients, etc., can solve the problems of undesired side effects, kill tumor cells, damage and death of cells, etc., and achieve good drug delivery effect and little side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1.1
[0063] Pluronic F-127 (PF-127) is a polymer having revserse thermal gelation. The early reports of Achmolka (Schmolka I R. Artificial skin. I. “Preparation and properties of pluronic F-127 gels for treatment of burns”, Journal of Biomedical Materials Research. 6(6):571-82, 1972) and the current results of other researchers (1. Morishita M. Barichello J M. Takayama K. Chiba Y. Tokiwa S. Nagai T., “Pluronic F-127 gels incorporating highly purified unsaturated fatty acids for buccal delivery of insulin”, International Journal of Pharmaceutics. 212(2):289-93, 2001; 2. El-Kattan A F. Asbill C S. Kim N. Michniak B B., “Effect of formulation variables on the percutaneous permeation of ketoprofen from gel formations”, Drug Delivery: Journal of Delivery & Targeting of Therapeutic Agents 7(3):147-53, 2000; 3. Onuki Y. Morishita M. Takayama K. Tokiwa S. Chiba Y. Isowa K. Nagai T., “In vivo effects of highly purfied docosahexaenoic acid on rectal insulin absorption”, International Journal of Ph...
example 1.2
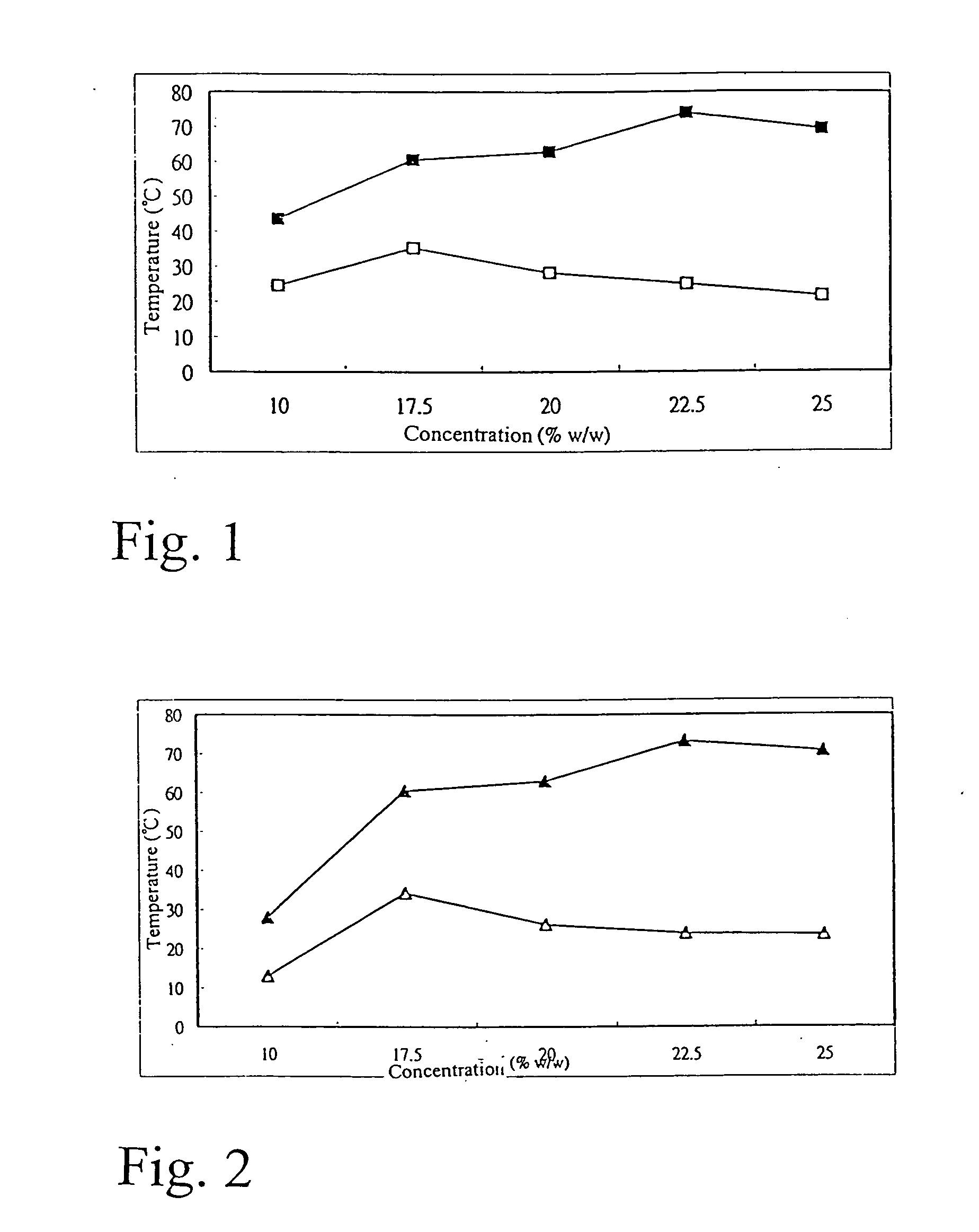
[0065] The sample and operation condition are as described in example 1.1 except that the specified amount of sample was cooled from 90° C. at a rate of 2° C. / min at a fixed volume and controlled temperature and at a speed of 400 rpm. The temperatures at which the rotor stoped (T2) and started revolution again (T1) were recorded, and the operation was stopped when the temperature was decreased to 4° C. The PF-127 was prepared into five concentration 10% w / w, 17.5% w / w, 20% w / w, 22.5% w / w, and 25% w / w. According to the procedure, the process was repeated three times to take the average, the results are shown in the Table 2 and FIG. 2.
TABLE 2PF-127 conc.(%, w / w)1st critical point(° C.)2nd critical point(° C.)10281317.560.4734.32062.9326.3322.573.23242570.723.67
example 1.3
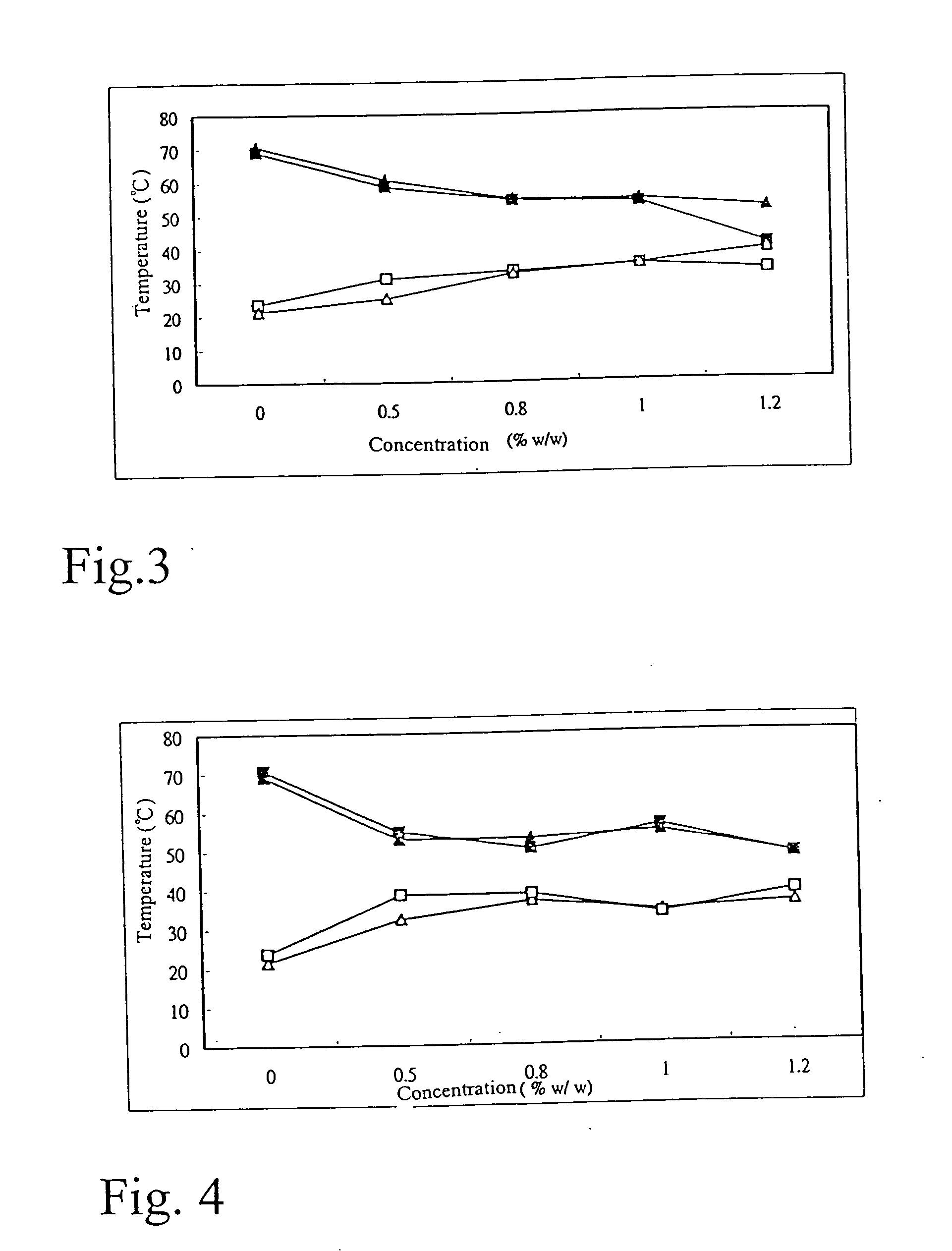
[0066] The sample and operation condition are as described in example 1.1 and example 1.2. The square represents the result of temperature increase test and triangle represents the result of temperature decrease test. The concentration of PF-127 was fixed at 25% w / w. Various concentrations of Carbopol 971P were added. Repeat the process three times and take the average. The results of the tests are shown in the Table 3 and FIG. 3.
TABLE 325% w / wPF-127 +Carbopol2nd crictical2nd crictical1st crictical1st crictical971P ofpoint in thepoint in thepoint in thepoint in theconc. (%temp.temp.temp.temp.w / w)increase testdecrease testincrease testdecrease test070.769.1323.6721.430.560.758.6731.1325.170.854.8354.3333.232.37154.253.2334.734.871.251.3740.432.7338.87
[0067] The test results have shown that the addition of mucoadhesive polymer Carbopol 971P will not affect the first critical point and second critical point of thermoresponsive polymer PF-127 significantly. Therefore, this is an excel...
PUM
| Property | Measurement | Unit |
|---|---|---|
| critical point | aaaaa | aaaaa |
| critical point | aaaaa | aaaaa |
| critical point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com