Method of inhibiting growth and proliferation of listeria monocytogenes using levulinate
a technology of listeria monocytogenes and levulinate, which is applied in the field of antibacterial methods, materials and methods, can solve the problems that the growth and proliferation of bacteria significantly affects the quality and safety of food, and achieve the effects of prolonging shelf life, stability and application of levulina
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Anti-Listeria Activity of Levulinate Added to Fresh Ready-to-Eat Meat
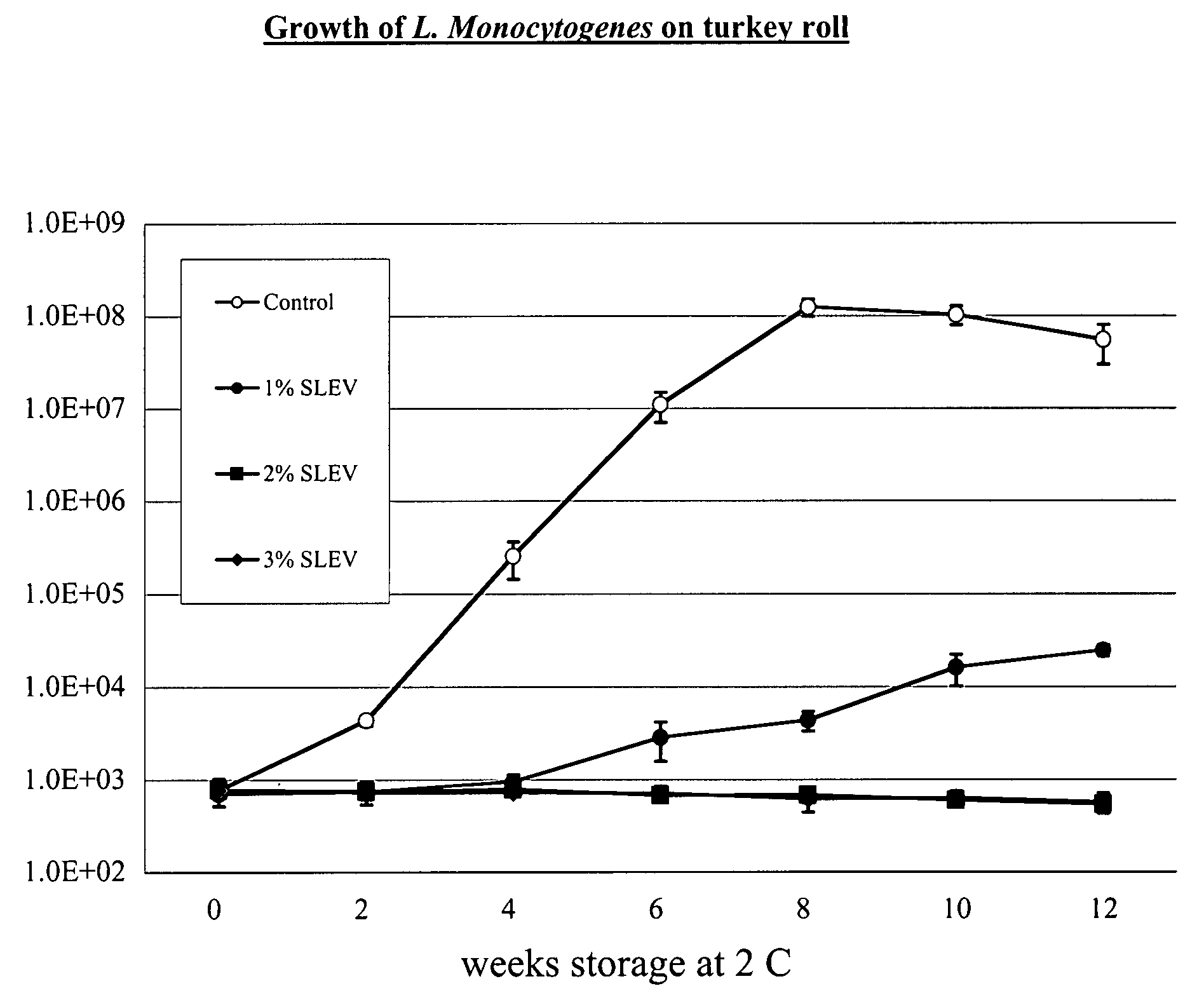
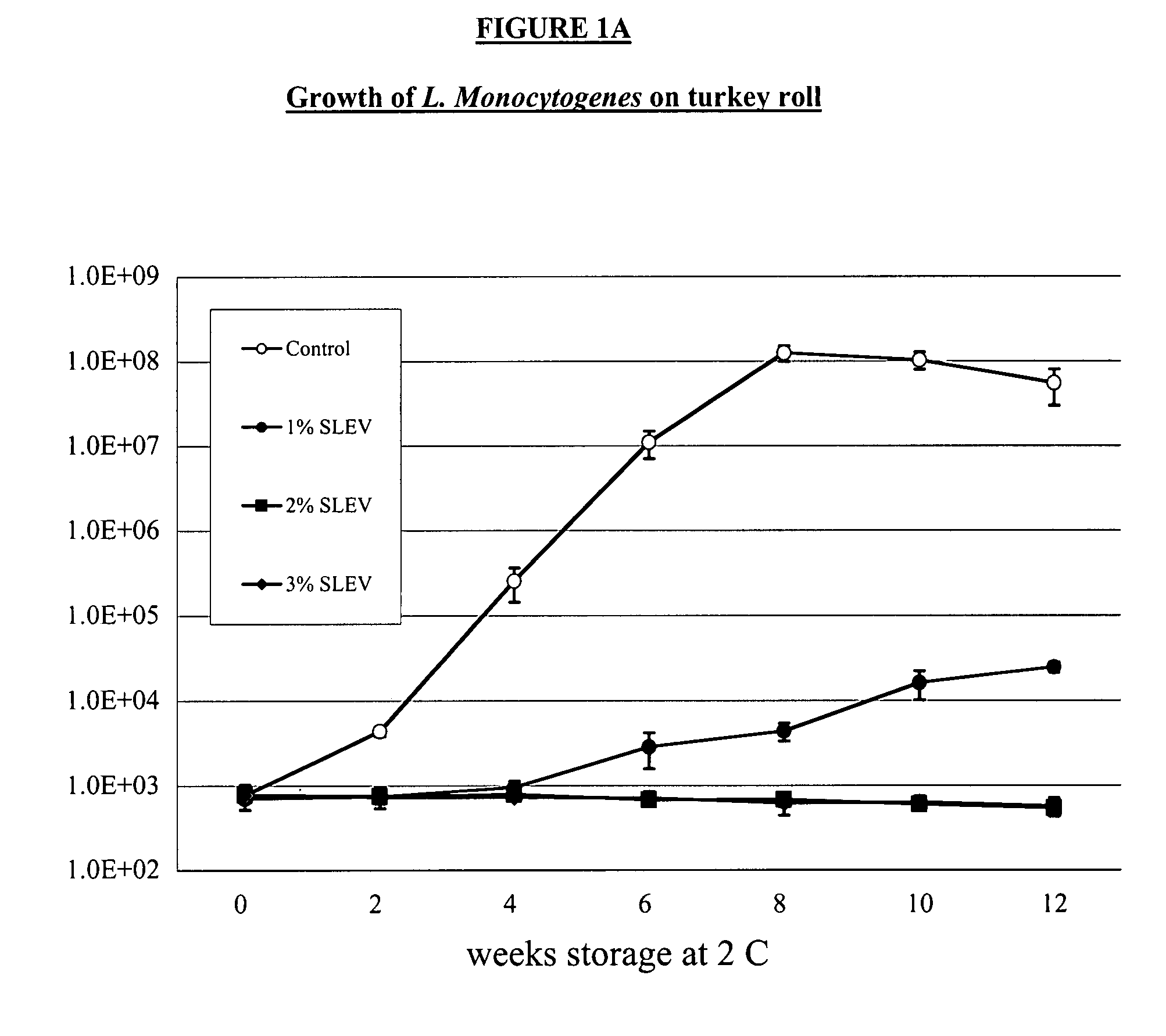
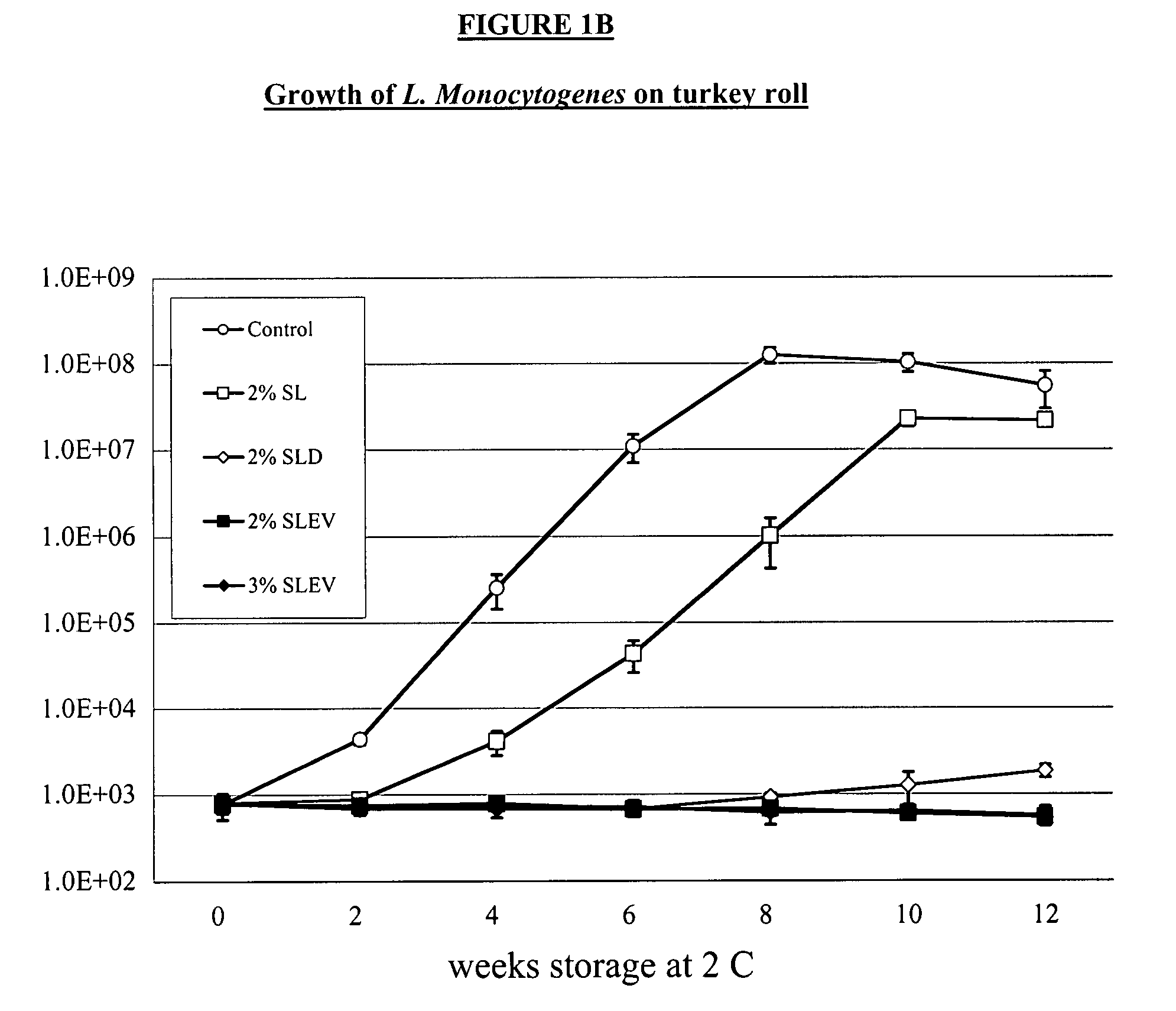
[0024]The following virulent strains of Listeria monocytogenes were obtained from International Life Sciences Institute (ILSI) collection housed at Cornell University (http: / / www.foodscience.cornell.edu / weidmand / listeriadbase.htm):[0025]FSL J1-177; ribotype DUP-1051D; lineage I; serotype 1 / 2b; isolated from human sporadic case.[0026]FSL C1-056; ribotype DUP-1030A; lineage II; serotype 1 / 2a; isolated from human sporadic case.[0027]FSL N3-013; ribotype DUP-1042B; lineage I; serotype 4b; food isolate associated with human listeriosis epidemic in the UK (1988-1990)[0028]FSL R2-499; ribotype DUP-1053A; lineage II; serotype 1 / 2a; human isolate associated with US outbreak linked to sliced turkey (2000).[0029]FLS N1-227; ribotype DUP-1044A; lineage I; serotype 4b; food isolate associated with US outbreak (1998-1999).
[0030]We completed two 12-week challenge trials in a ready-to-eat product made from turkey breast. Product w...
example 2
Anti-Listeria Activity of Levulinate Added to Cured Ready-to-Eat Meat
[0033]We completed two separate 12-week challenge trials in a cured ready-to-eat bologna made from pork. Product was formulated, and a volume of 60% sodium levulinate syrup was contacted with the meat by direct addition and bowl chopping of a 30% sodium levulinate solution sufficient to obtain final concentrations (v / wt of meat) of 1%, 2%, and 3% sodium levulinate. Product was stuffed into casings and fully cooked to 71° C., and the final product was sliced. Sample slices were then inoculated with the 5 strain cocktail of L. monocytogenes (described in Example 1), vacuum packaged, and stored at 2° C. for up to 12 weeks. Samples were collected biweekly and analyzed for growth of L. monocytogenes using BioRad Rapid L'mono.
[0034]The results from this experiment are shown in FIG. 2A. Counts of L. monocytogenes recovered from untreated control bologna showed significant growth from the inoculated level after 6 weeks of ...
example 3
Effect of Sodium Levulinate on Taste Sensation in a Taste Panel
[0036]A sensory panel consisting of anonymous consumers rated samples of turkey breast roll and bologna for their overall liking of the products. Samples were scored on a scale of 1 to 9, with 1=strongly dislike, 5=neither like nor dislike, and 9=strongly like. In the turkey roll panel, judges were given samples containing no antimicrobial (control), 2% sodium lactate, 2% sodium lactate plus sodium diacetate, 2% sodium levulinate, and 3% sodium levulinate. In the bologna analysis, the consumers were given samples containing no antimicrobial (control), 2% sodium lactate, the 2% combination of sodium lactate and sodium diacetate, 1% sodium levulinate and 2% sodium levulinate. There were 132 consumers that participated in the sensory panel for the turkey roll and 112 for the bologna. Of those who participated in the turkey roll panel, 66 were female and 66 were male. There was a wide range of age among the participants, wit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com