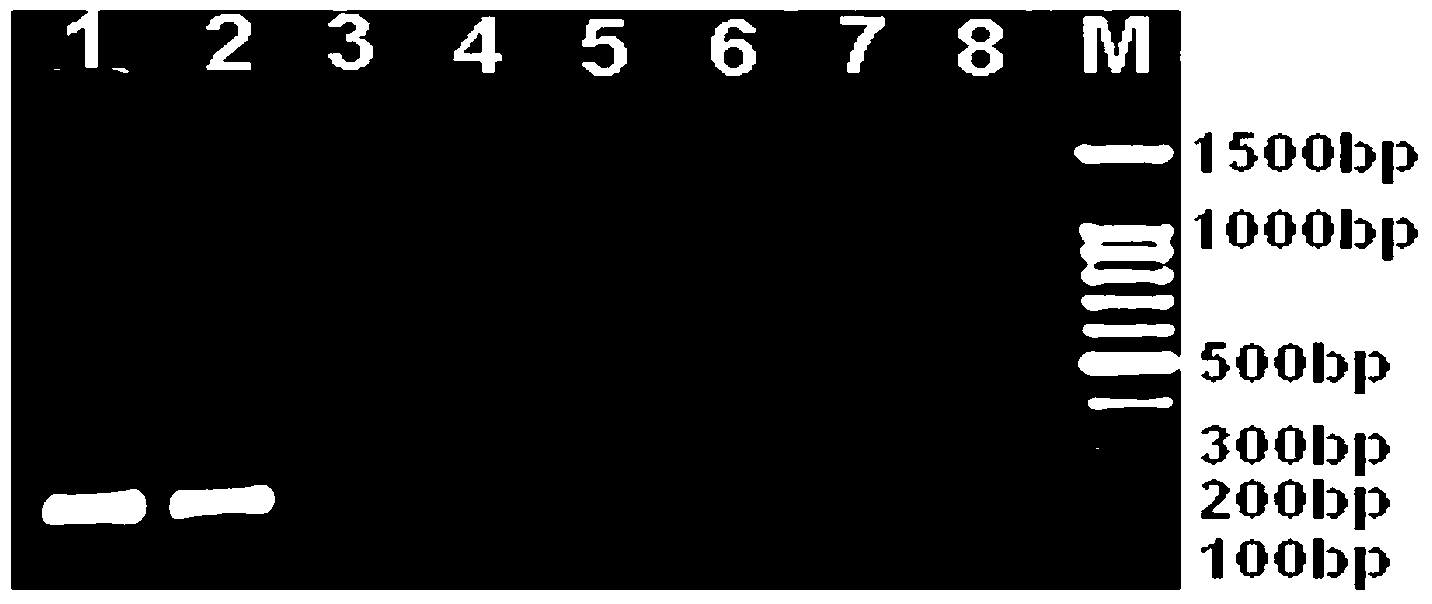Patents
Literature
46 results about "Karyocytomegaly" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
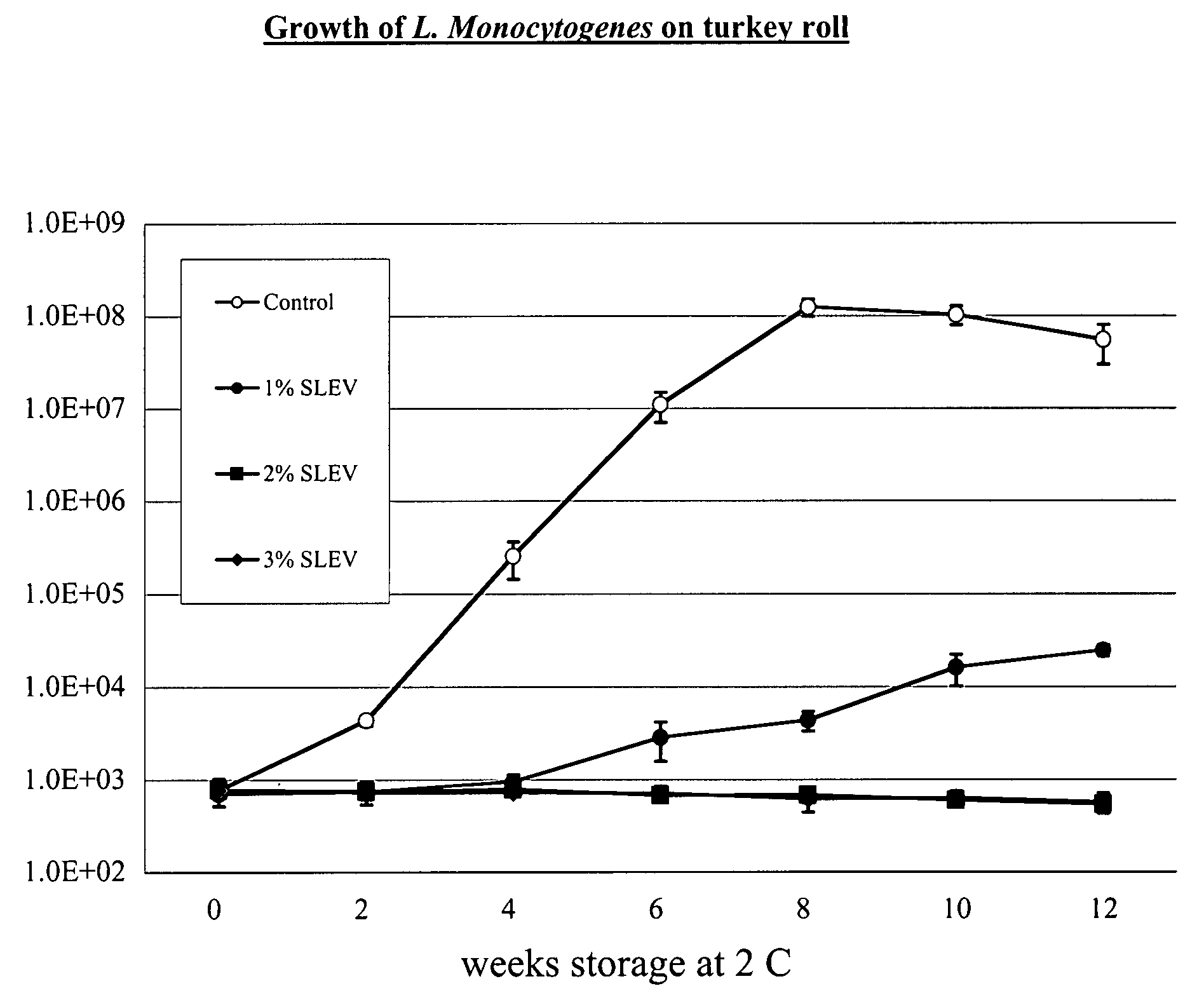
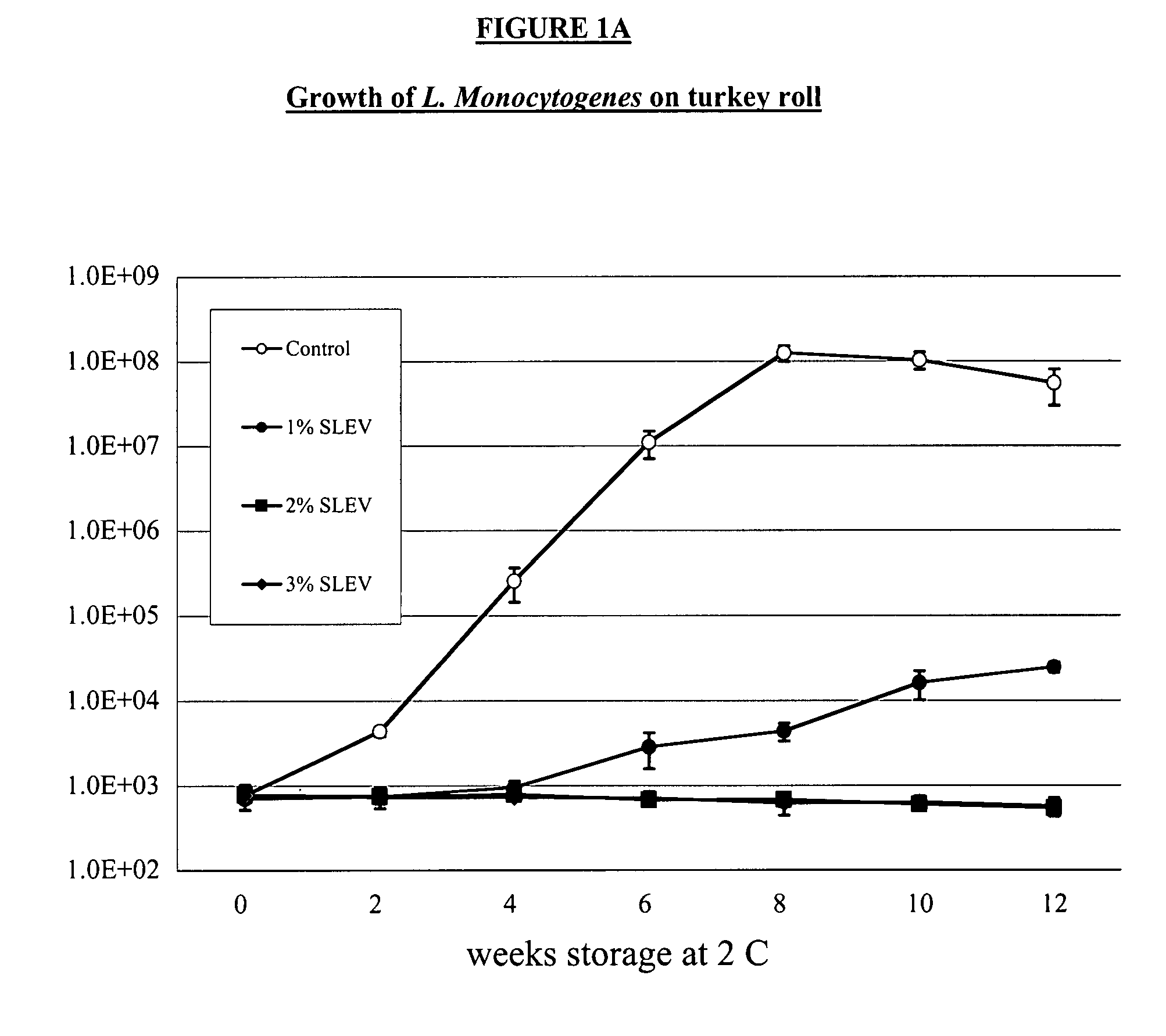
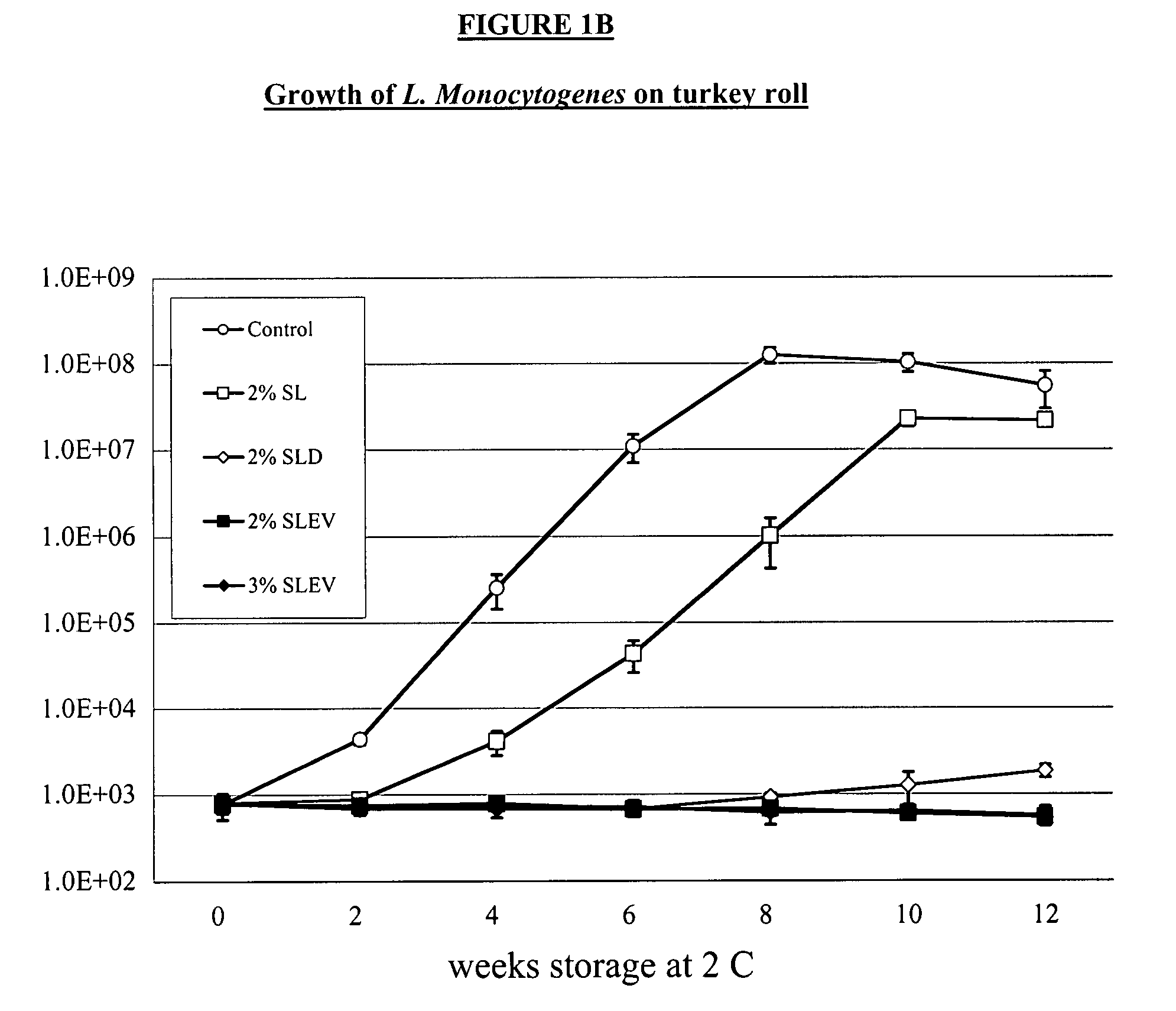
System for handling processed meat and poultry products
InactiveUS6964788B2Apparent advantageObvious advantagesMeat/fish preservation using liquidsSausage casingsPathogenic microorganismPoultry product
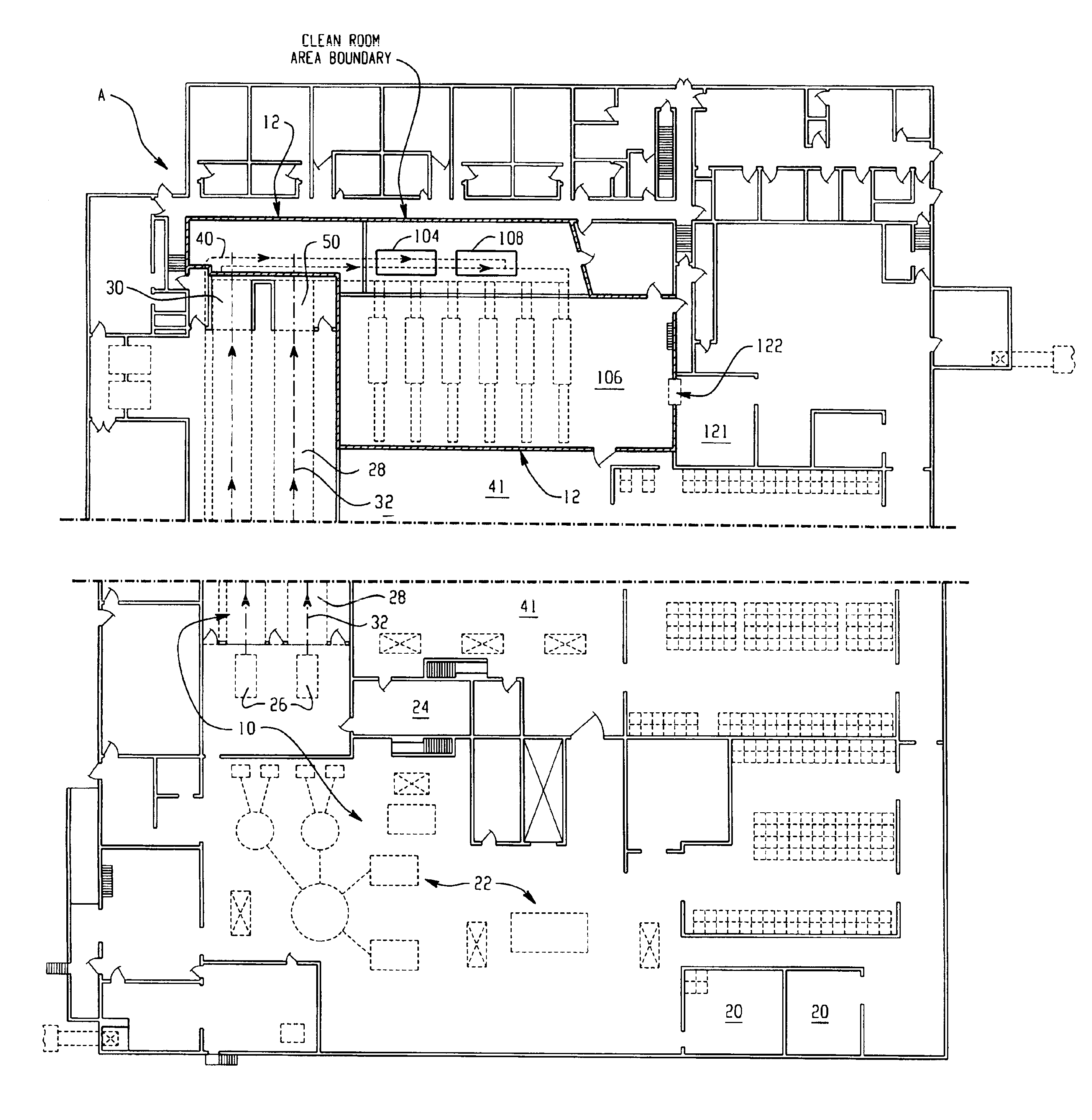
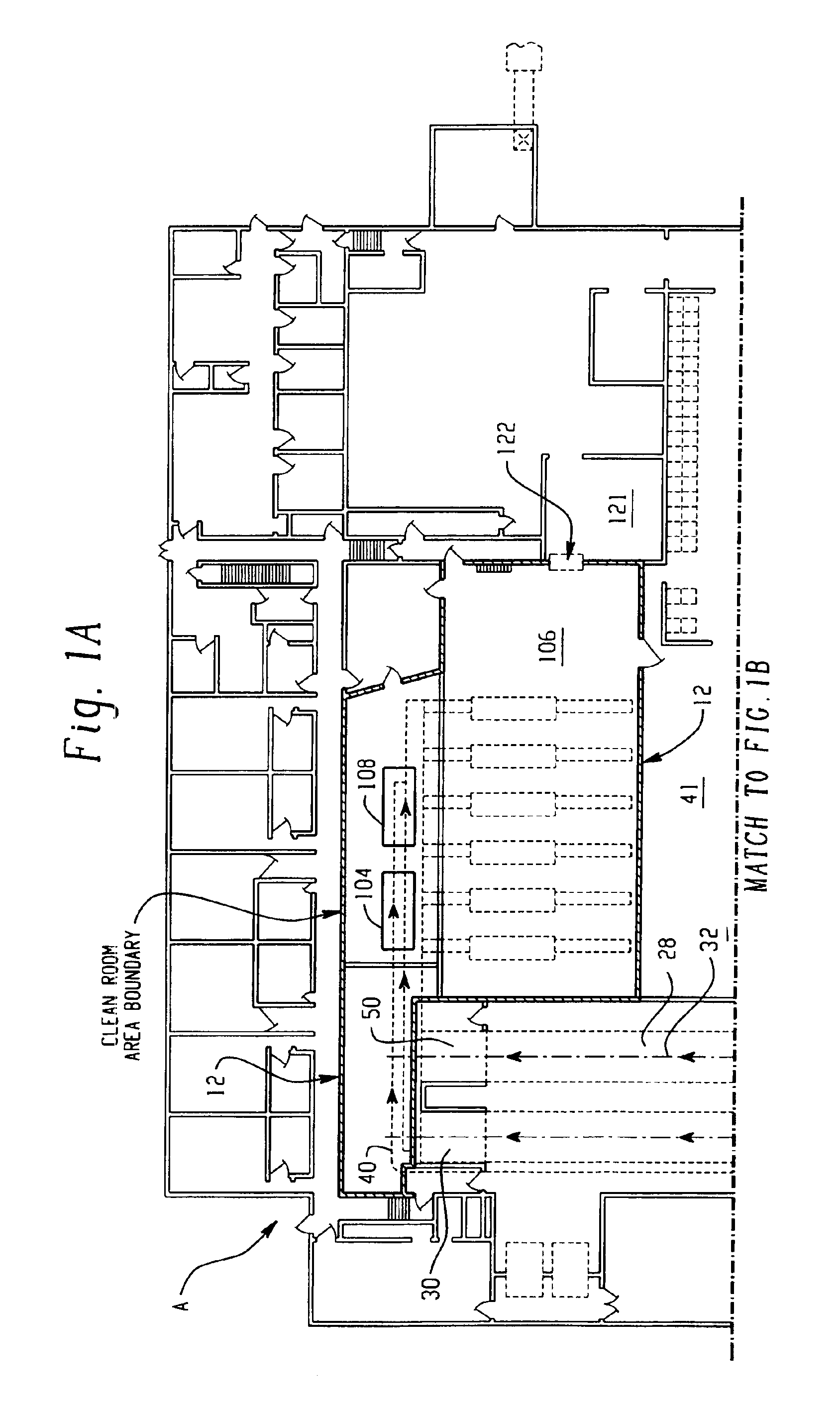
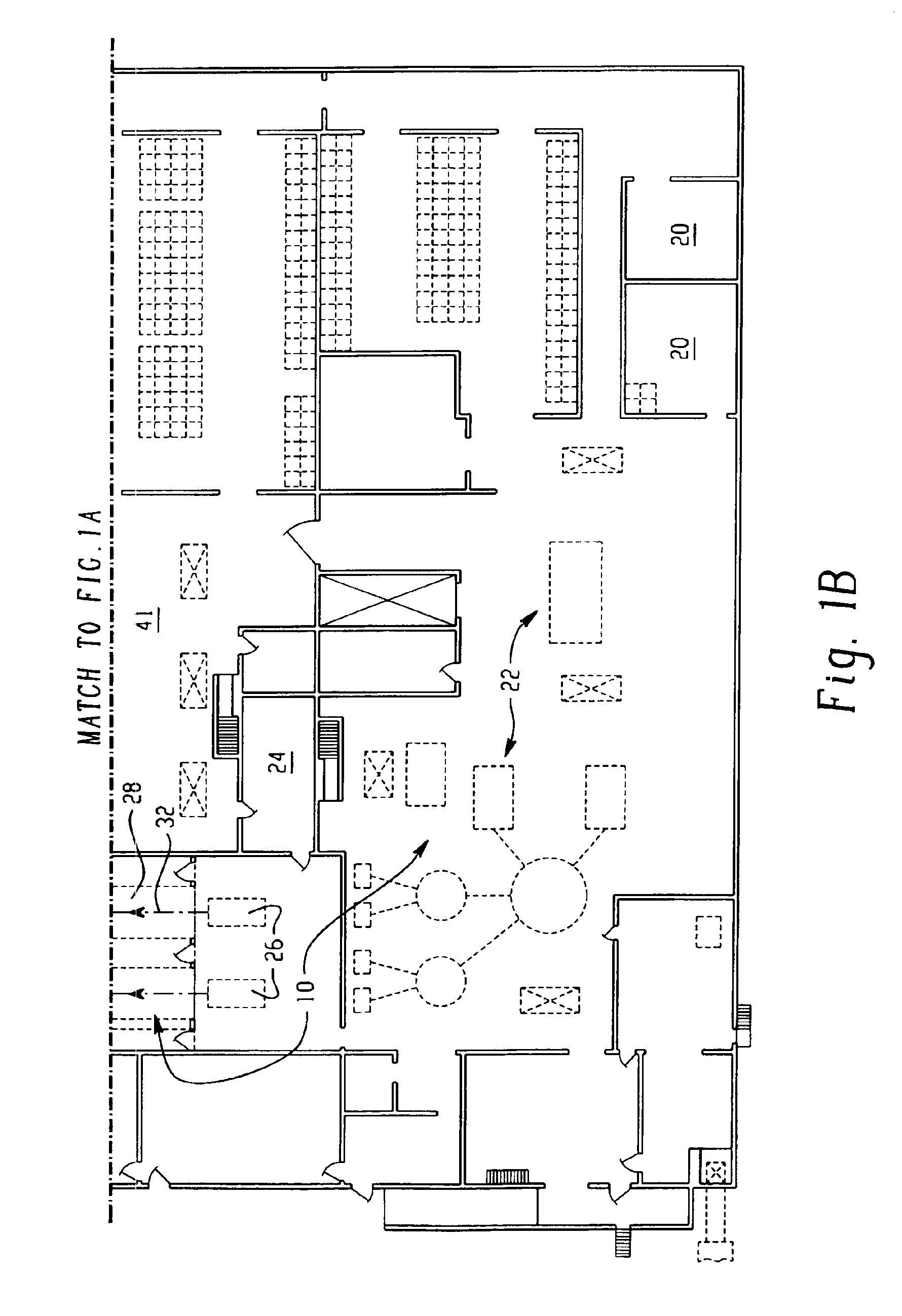
Food products, such as precooked meats, sausages, and the like are microbially decontaminated in their cooking packages to remove surface microorganism contamination from the packages. The cooking packages are removed and the food products optionally subjected to further processing, such as slicing, and then packaged in aseptic packaging. The microbial decontamination step, further processing and packaging are carried out in a clean room which is maintained to a high level of sterilization or disinfection to minimize or eliminate contamination of the food products with pathogenic microorganisms such as Listeria Monocytogenes. The food products thus leave the packaging plant with a much higher assurance of food safety than is found in a conventional packaging plant.
Owner:STERIS CORP
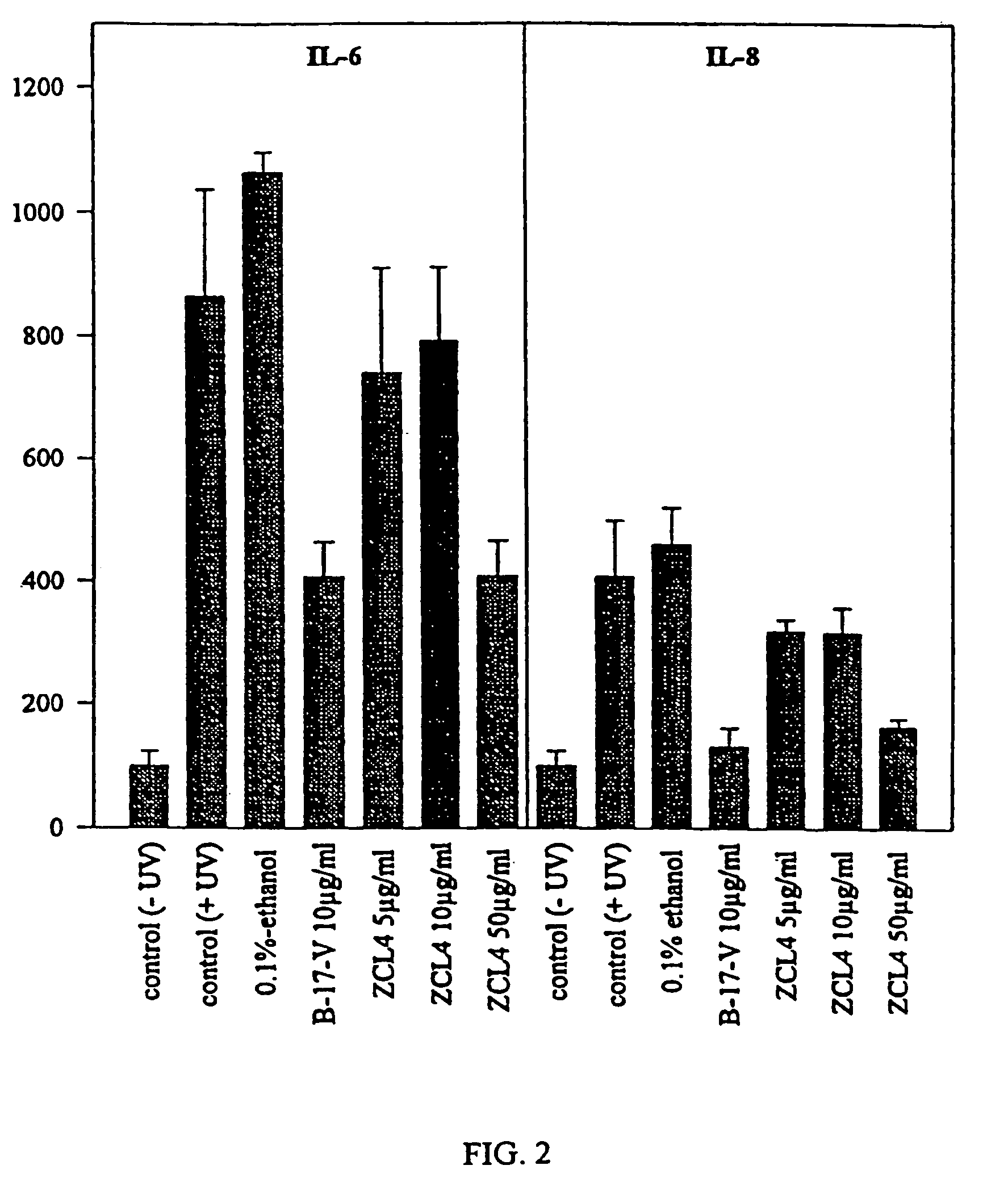
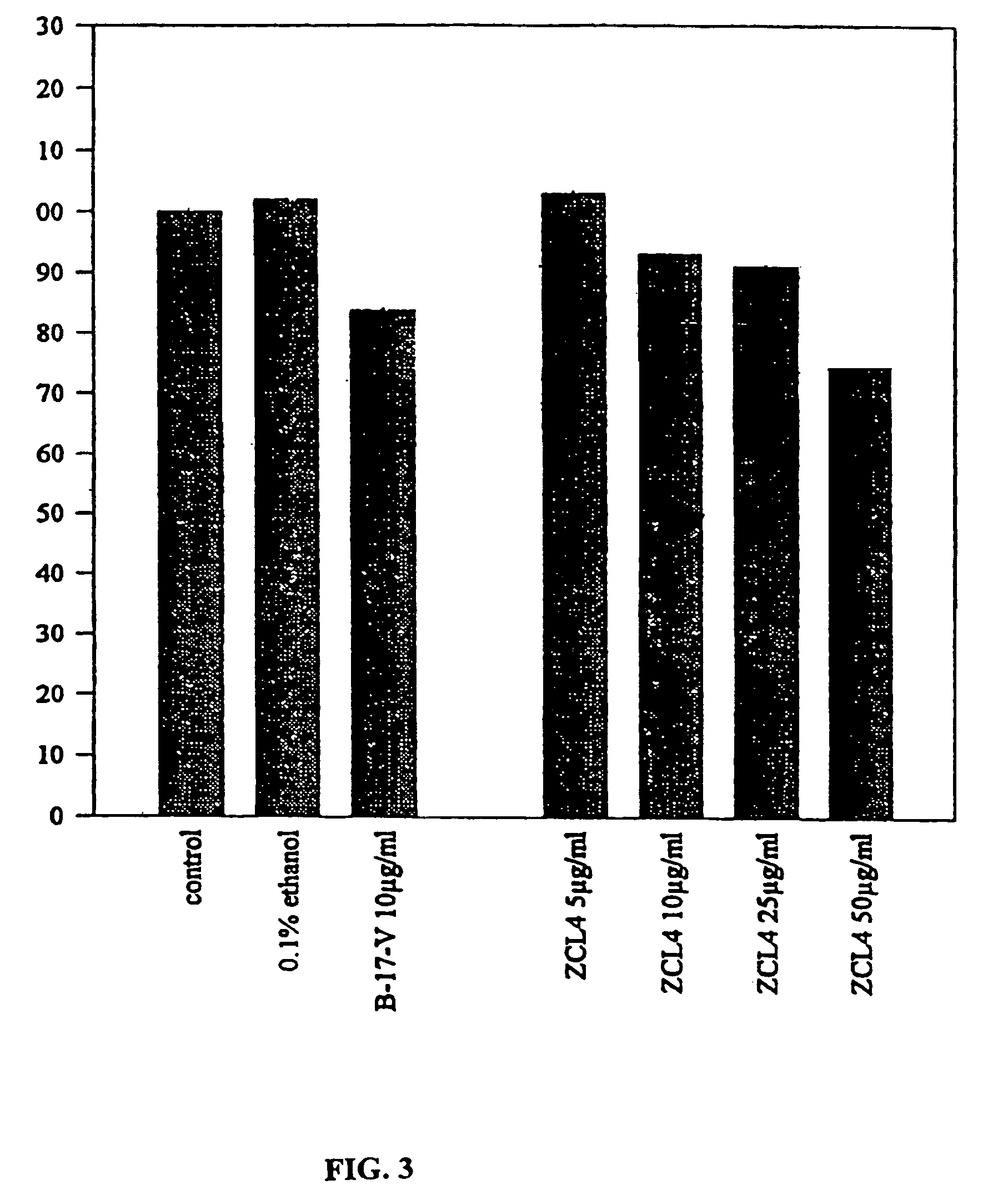
Pharmacological activities of Curcuma longa extracts
InactiveUS7220438B2Increase proliferative activityHigh activityBiocideDrug compositionsCurcuma longa extractWater soluble
This invention concerns to a topical pharmaceutical composition comprising an water soluble Curcuma extract, and suitable excipients for said topical administration; the process for obtaining said pharmaceutical compositions; the use of different Curcuma extracts as photosensitizing agents for the treatment of proliferative diseases; and the use of Curcuma extract or curcuminoids in combination with a radiation for the treatment of proliferative diseases on eukaryote cells.
Owner:ASAC COMPANIA DE BIOTECHA E INVESTIGACION
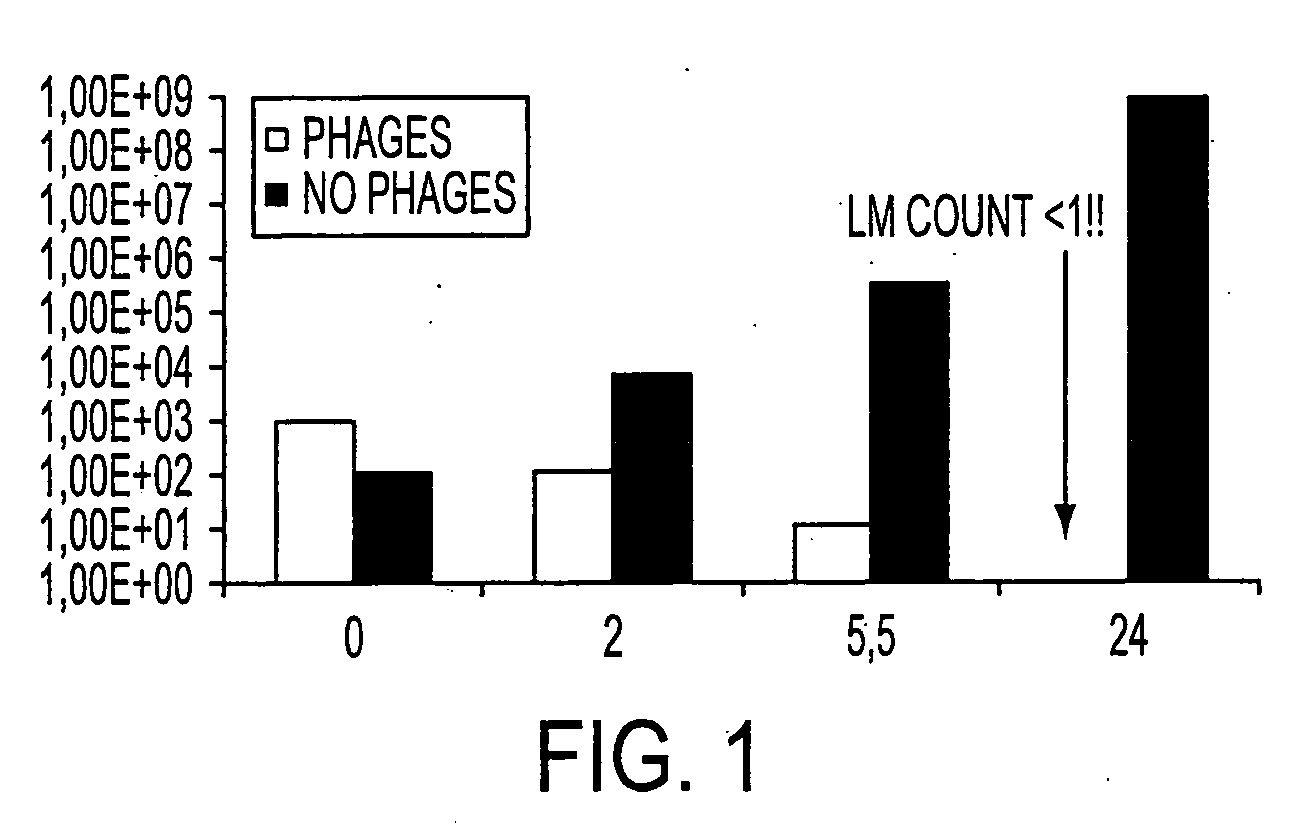
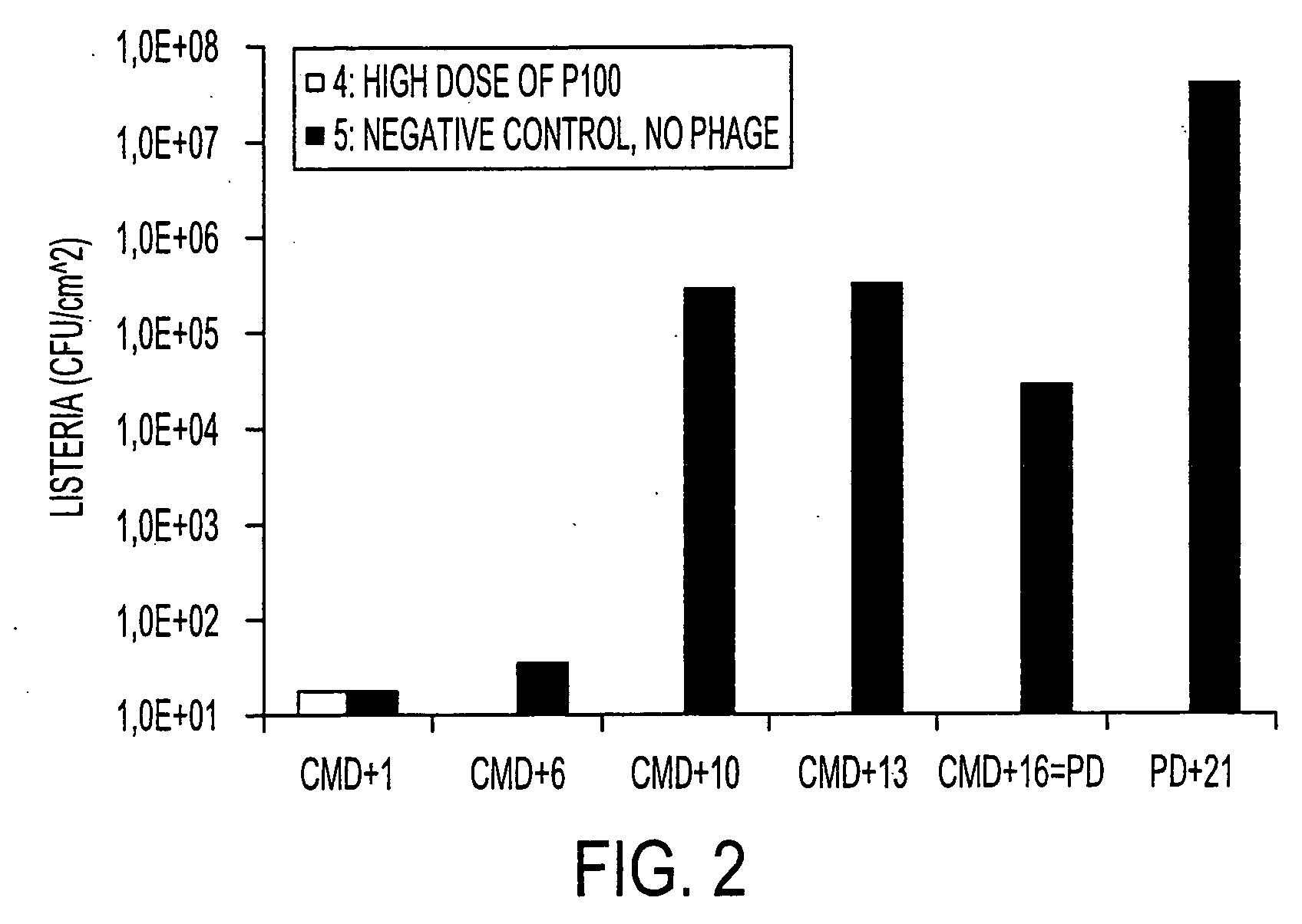
Virulent phages to control listeria monocytogenes in foodstuffs and in food processing plants
ActiveUS20050175594A1Shorten the counting processGrowth inhibitionAntibacterial agentsBiocideBiotechnologyBacteroides
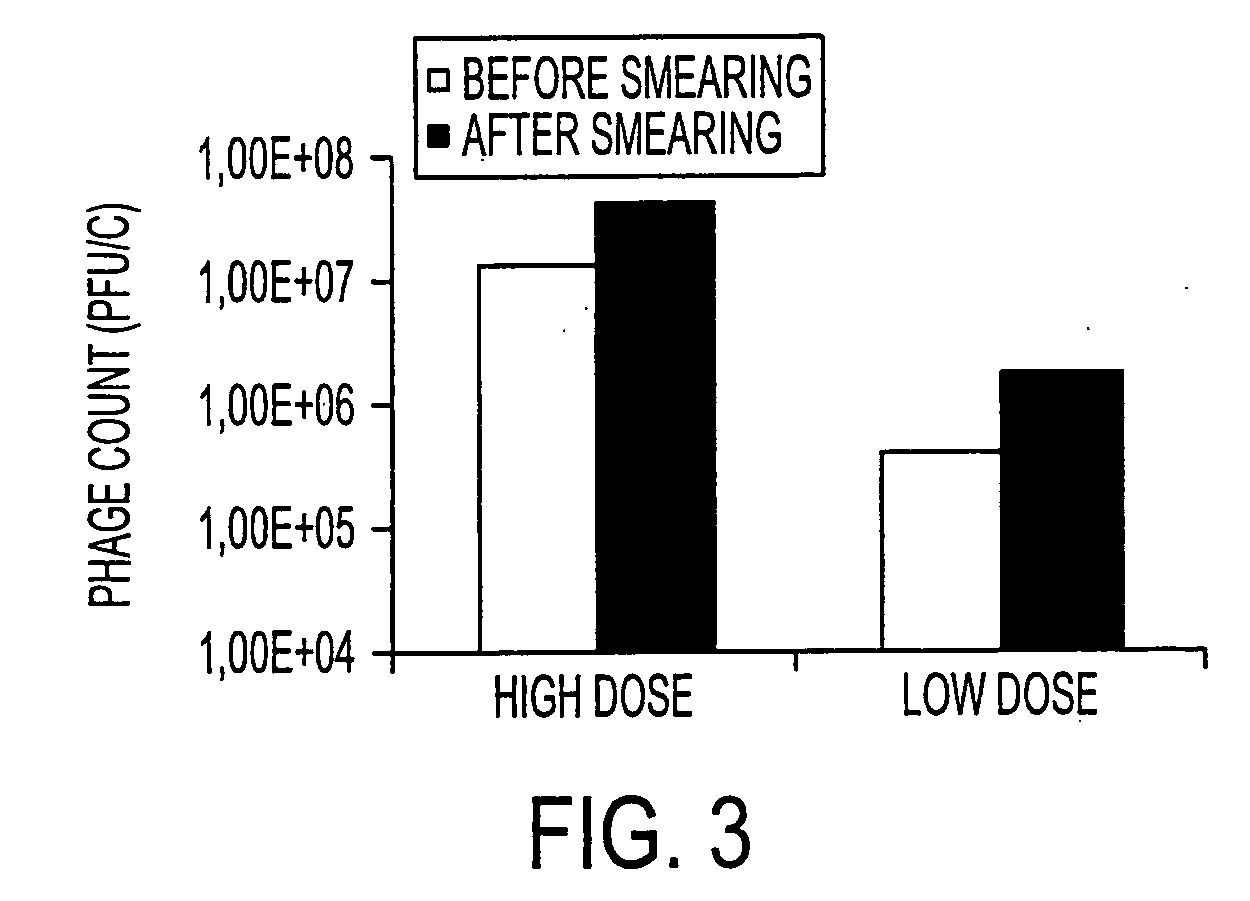
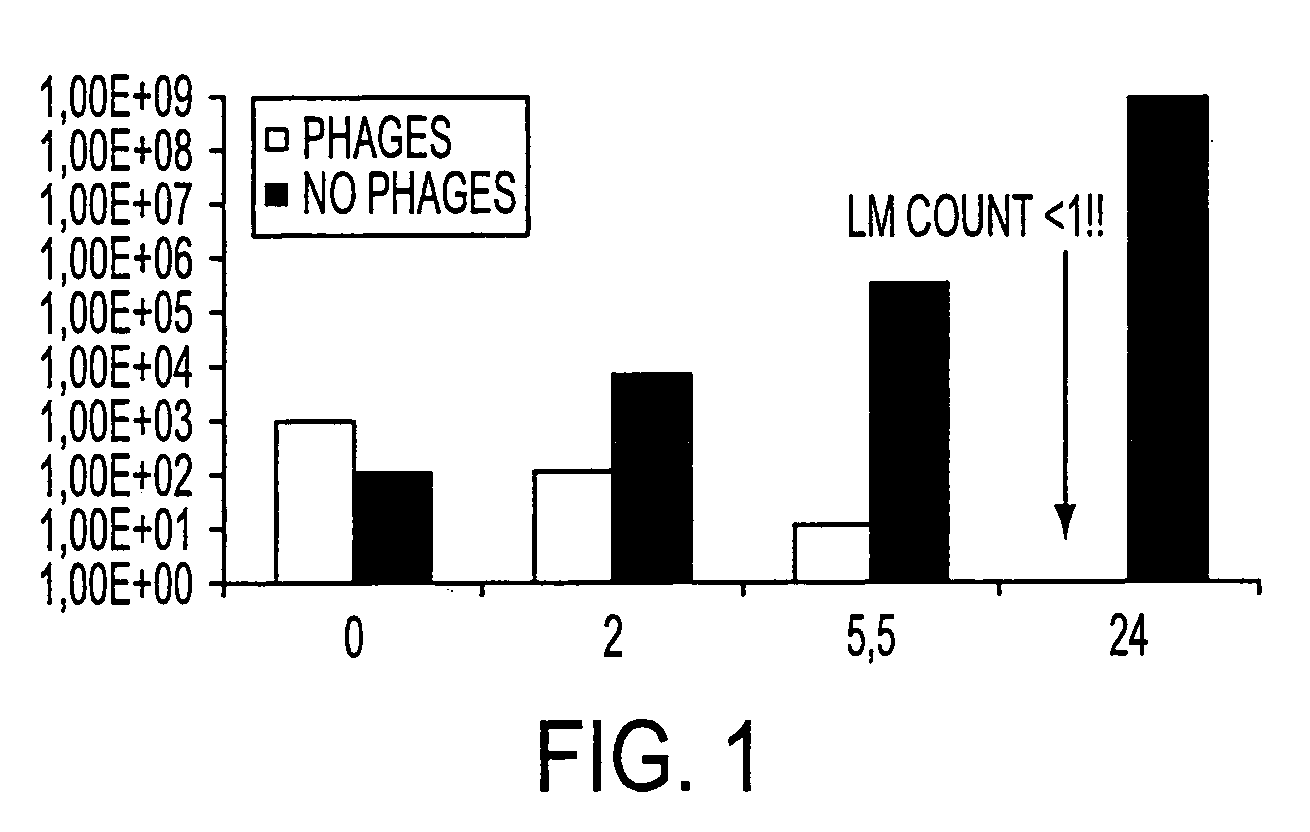
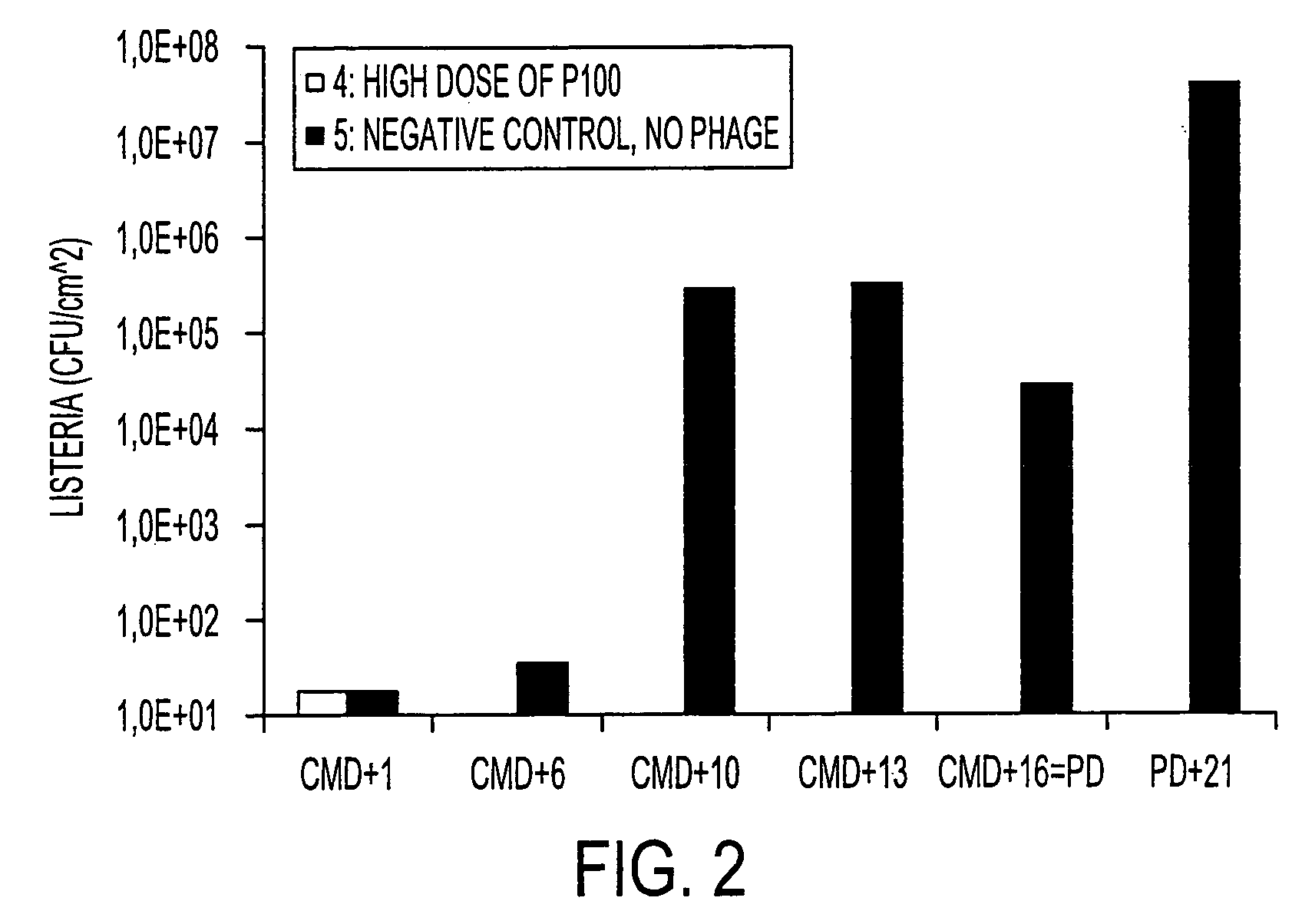
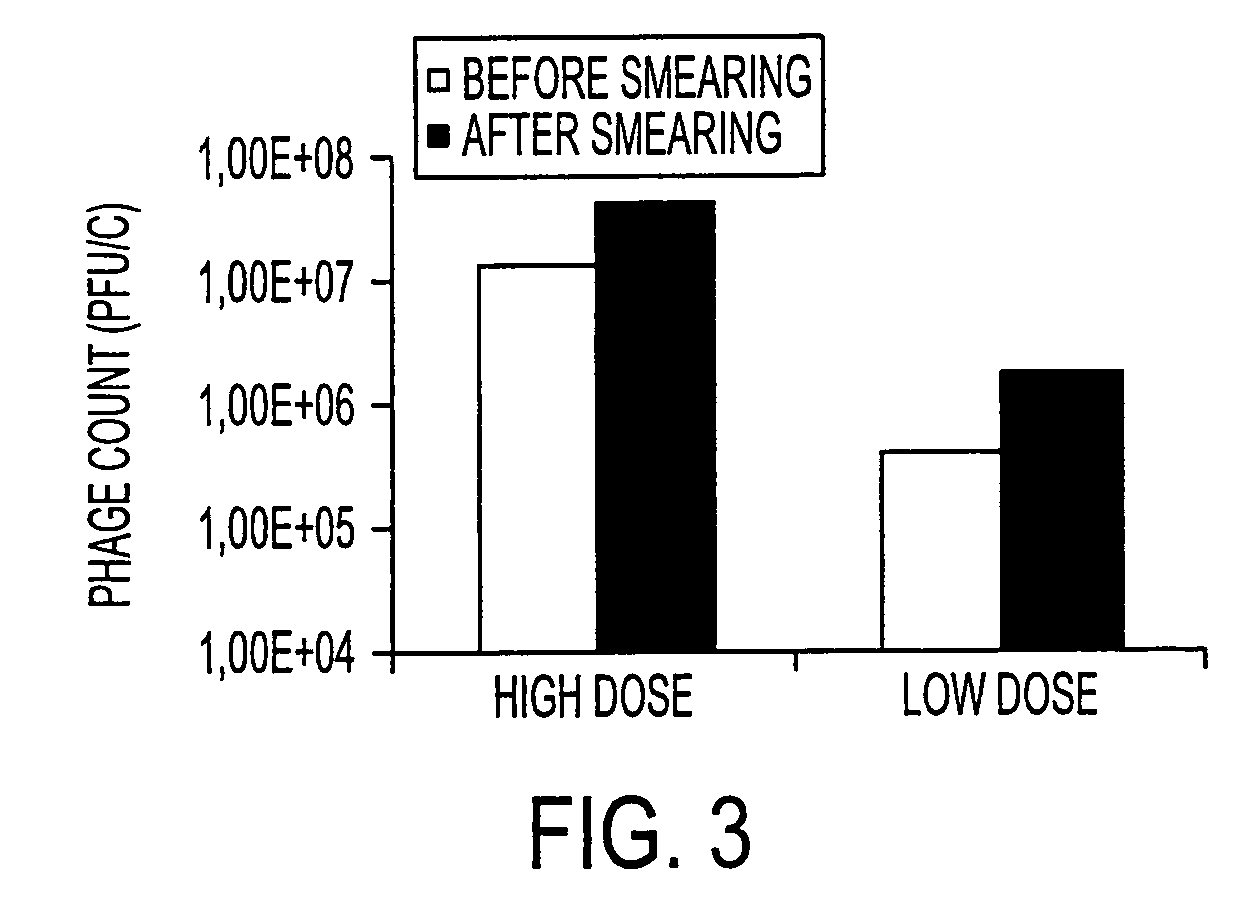
The present invention relates to virulent (lytic) Listeria monocytogenes phage from the Myoviridae family, preferably P100, alone or in combination with other virulent phages. P100 and the endolysin from P100 can be administered to food products, to the components that will be added to food products, and / or to the infrastructure of the food processing plants within which such food products are processed, or the containers or wraps in which such foods are stored and / or shipped, in order to reduce Listeria monocytogenes contamination. P100 can also be used in the present invention to identify Listeria monocytogenes bacteria present on (or within) foodstuffs, as well as those Listeria monocytogenes bacteria present in the equipment or the general environment of the food processing plants in which the foodstuffs are being processed and in animals infected with Listeria monocytogenes. The phage and the endolysin of the present invention can also be used to treat animals infected with Listeria monocytogenes. P100 will kill the bacteria that are within its host range with great efficiency and will propagate to high titer thereon. P100 can be combined with other lytic phage, and / or with other antimicrobial agents to reduce or eliminate Listeria.
Owner:EBI FOOD SAFETY
LAMP method for detecting real-time turbidity of Listeria monocytogenes
InactiveCN104313173AMicrobiological testing/measurementMicroorganism based processesTurbidityMicrobiology
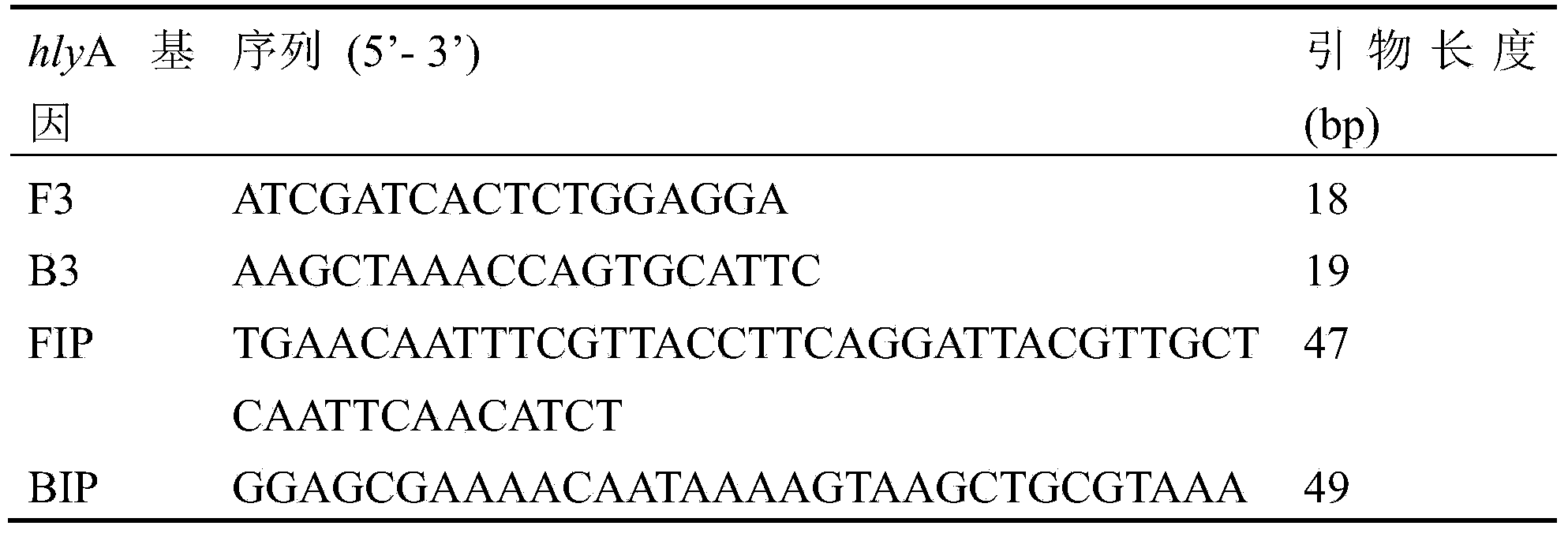
The invention aims at providing an LAMP method for rapidly detecting the real-time turbidity Listeria monocytogenes, for making up the defects of the prior art. The method comprises the following step: firstly, providing an LAMP primer group for detecting Listeria monocytogenes, wherein the nucleotide sequences of the LAMP primer group are as shown in SEQ ID NO:1-4. A set of LAMP primers are designed aiming at the hemolysin gene of the Listeria monocytogenes, a real-time turbidity instrument is adopted to detect the Listeria monocytogenes, and the specificity and the sensitivity of the method are verified, so that a rapid, specific and sensitive Listeria monocytogenes LAMP detection method is established.
Owner:ZHOUSHAN INST OF CALIBRATION & TESTING FOR QUALITY & TECHNICAL SUPERVISION
Method of loop-mediated isothermal amplification (LAMP) for detecting Listeria monocytogenes
InactiveCN101748201AHigh reaction specificityStrong specificityMicrobiological testing/measurementMicroorganismLoop-mediated isothermal amplification
The invention discloses a method of loop-mediated isothermal amplification (LAMP) for detecting Listeria monocytogenes. At present, the traditional detection method for microorganisms has complex operation, besides, the detection time is longer with 5-15 days of culture and identification time required generally and the requirement of rapid detection cannot be met on time. The method comprises the following: pretreatment, detection process, result detection and result judgment, target gene of the Listeria monocytogenes is selected (the GenBank accession number AF532235), two pairs of primers are designed, the LAMP gene amplification is carried out for the nucleic acid of the Listeria monocytogenes through providing a specific primer group, a specific gene fragment is detected whether to exist in the sample, and further the Listeria monocytogenes are determined whether to exist in the sample. The method is used in detection of foodborne bacterial pathogens.
Owner:INSPECTION & QUARANTINE TECH CENT OF HEILONGJIANG ENTRY EXIT INSPECTION & QUARANTINE BUREAU OF P R C
Pharmacological activities of Curcuma longa extracts
InactiveUS20040126442A1High activityDifferent typeBiocideUnknown materialsCurcuma longa extractBuccal administration
This invention concerns to a topical pharmaceutical composition comprising an water soluble Curcuma extract, and suitable excipients for said topical administration; the process for obtaining said pharmaceutical compositions; the use of different Curcuma extracts as photosensitizing agents for the treatment of proliferative diseases; and the use of Curcuma extract or curcuminoids in combination with a radiation for the treatment of proliferative diseases on eukaryote cells.
Owner:ASAC COMPANIA DE BIOTECHA E INVESTIGACION
Aptamers that bind to listeria surface proteins
Aptamers bind to Listeria surface proteins. A method of assaying a sample for the presence of Listeria monocytogenes includes exposing the sample to an aptamer that specifically binds one of the following proteins: Listeria monocytogenes internalin A protein, Listeria monocytogenes internalin E protein, and Listeria monocytogenes 0610 protein. The presence of Listeria monocytogenes in the sample is detected when the aptamer binds the protein present in the sample. A method of treating Listeria monocytogenes infection includes administering the aptamers to the mammal at a concentration sufficient to reduce Listeria monocytogenes infection.
Owner:RESONAC CORPORATION +1
Nucleic acid aptamer capable of specifically recognizing Listeria monocytogenes, screening method and application thereof
ActiveCN102010865AEasy to operateLower synthesis costMicrobiological testing/measurementDNA preparationFluoresceinScreening method
The invention discloses a nucleic acid aptamer capable of specifically recognizing Listeria monocytogenes, a screening method and application thereof. By using the SELEX (Systematic Evolution of Ligands By Exponential Enrichment) technology, a single-chain DNA oligonucleotide aptamer capable of specifically recognizing Listeria monocytogenes is obtained, and the aptamer can be transformed into a reporting aptamer by means of fluorescein labels and the like to be used for detecting Listeria monocytogenes. The sequence of the aptamer has wide application prospects in the aspect of accurately and fast detecting Listeria monocytogenes in food.
Owner:JIANGNAN UNIV
Method for checking monocyte hyperplasia Listeria
The invention discloses a method for detecting viable Listeria monocytogene, relating to a method for detecting Listeria. The invention solves the problem that the prior method for detecting viable Listeria monocytogenes is easy to produce false positive. The method for detecting viable Listeria monocytogenes is carried out as following steps: after extracting bacterial sludge from articles to be detected, extracting RNA to implement retrotransposon and real time PCR; accordingly, a collection of illustrative plates which are capable of determining whether the articles contain viable Listeria monocytogenes or not. The method has the advantages of high sensitivity, good specificity, simple operation, and comparatively short period. The method meets the requirements of pathogen rapid detection in food industry.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Capillary-column-based bioseparator/bioreactor with an optical/electrochemical detector for detection of microbial pathogens
InactiveUS8211657B2Peptide/protein ingredientsMicrobiological testing/measurementHigh concentrationElectrochemical detector
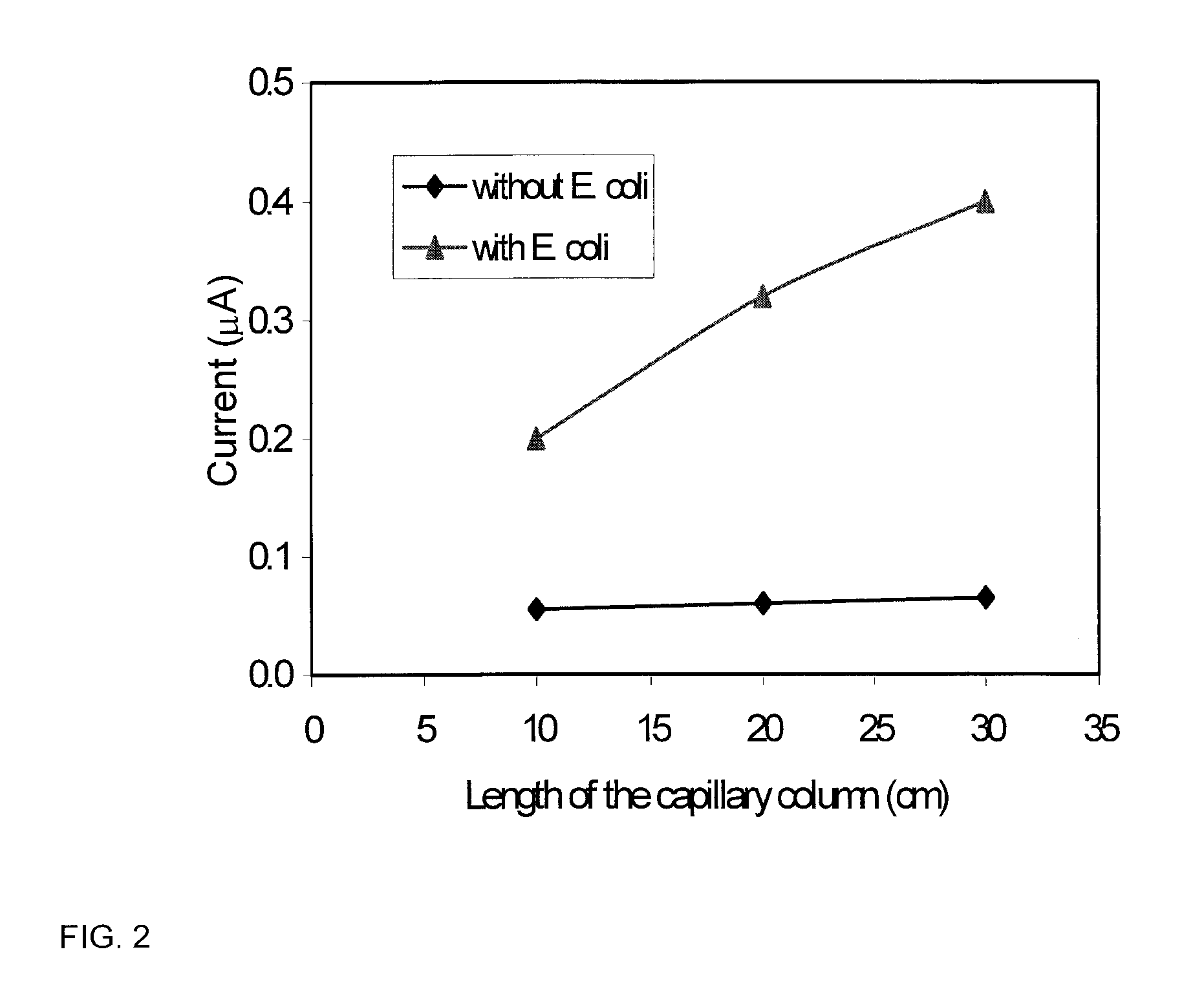
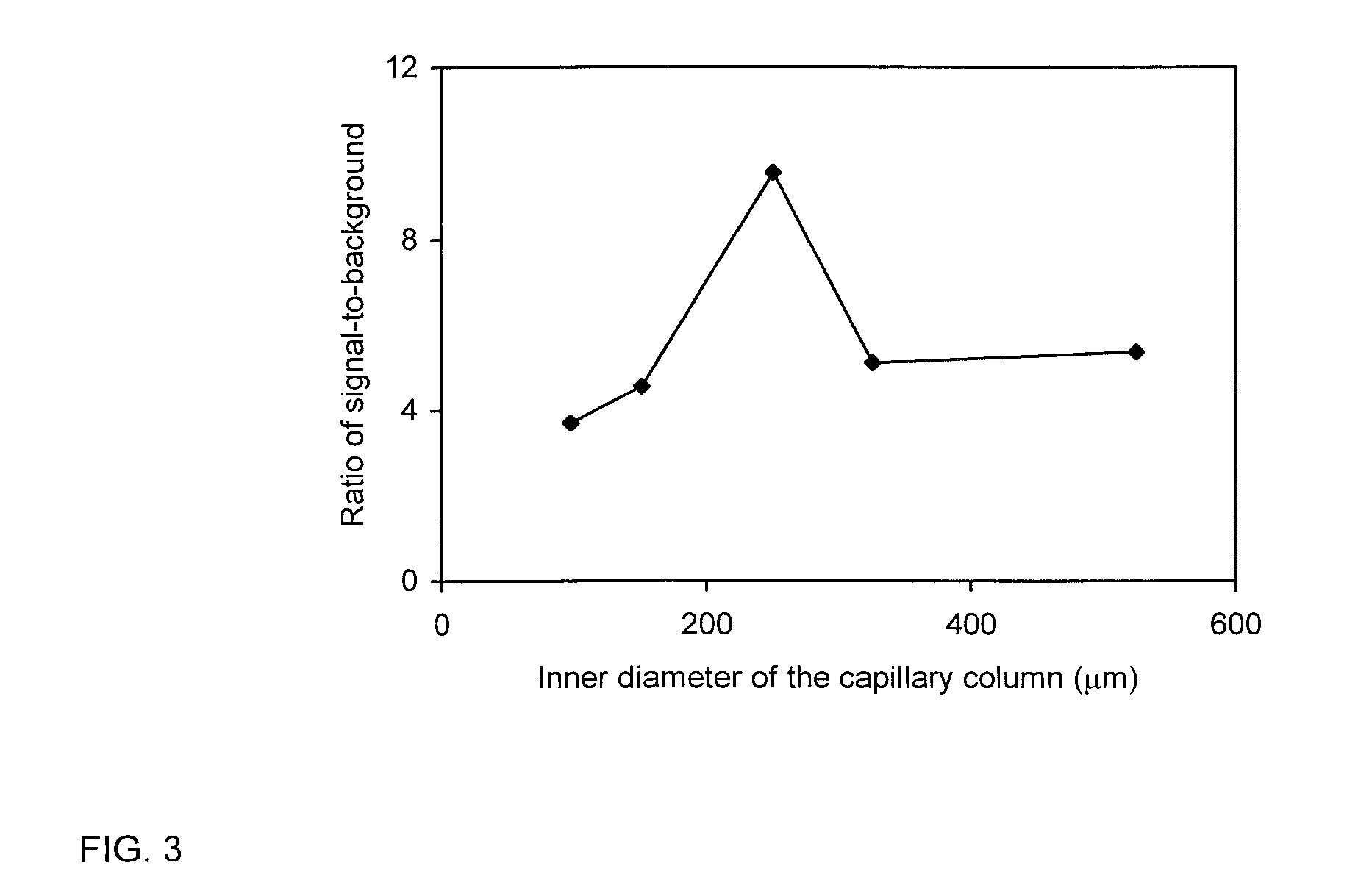
The present invention is directed to satisfying the need to detect microbial contamination of food products. The described bioseparator / bioreactor coupled with an optical / electrochemical biosensor was able to specifically detect E. coli O157:H7 from 8.8×101 to 8.8×106 CFU / ml in 2.5 hours without any enrichment. Using this invention, concentrations of S. Typhimurium ranging from 8.6×102 to 8.6×106 CFU / ml in pure culture were detected in 2 hours without any enrichment. The invention may also be used for the detection of S. Seftenberg, which has the same sensitivity as S. Typhimurium. Other pathogens such as L. monocytogenes and S. Heidleberg did not interfere with the detection. The optimum inner diameter of the 25 cm long column for the detection of E. coli O157:H7 is 250 μm. The detection limit for other microbial pathogens may be controlled by changing the length of capillary columns, using higher concentration of the labeled antibodies, altering the flow rate and concentration of the substrate, and increasing the reaction temperature to 37° C.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS
Method of inhibiting growth and proliferation of listeria monocytogenes using levulinate
InactiveUS20080045592A1Extended shelf lifeImprove stabilityBiocideDisinfectantsBacteroidesLevulinic acid
Methods, formulations and kits containing levulinate or levulinic acid are disclosed that have potent and unexpected properties to inhibit the growth and / or proliferation of Listeria monocytogenes bacteria in a food sample.
Owner:UTAH STATE UNIVERSITY
Nucleotide sequence for detecting listeria monocytogenes and detection method and detection kit
InactiveCN104450940AStrong specificityHigh sensitivityMicrobiological testing/measurementMicroorganism based processesMicrobiologyNucleotide sequencing
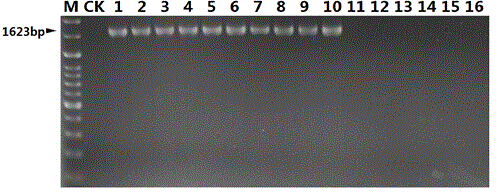
The invention discloses a nucleotide sequence for detecting listeria monocytogenes and a detection method and a detection kit, wherein the nucleotide sequence is expressed as SEQ ID NO. 1, the detection method is as follows: firstly, extracting the gene group DNA of the sample to be detected; secondly, taking the gene group DNA as the template, mixing with the specific primer Lm16, PCR buffer solution, MgCl2 solution, deoxidation nucleoside triphosphate mixture and Taq-DNA polymerase for preparing the PCR reaction system, carrying out the PCR reaction; finally, detecting whether the PCR product is the single amplification product, the size of which is 1623bp. The kit comprises the specific primer, PCR buffer solution, MgCl2 solution, deoxidation nucleoside triphosphate mixture and Taq-DNA polymerase. The kit can effectively detect the listeria monocytogenes in the food, and the detection sensitivity for the bacteria is high, the specificity is good and the antijamming capability is strong.
Owner:NANJING AGRICULTURAL UNIVERSITY
Listeria monocytogenes bacteriophage and uses thereof
The present invention is directed to isolated Listeria monocytogenes bacteriophage, and methods of using Listeria monocytogenes bacteriophage, or polynucleotides and polypeptides derived therefrom, to control the growth or contamination of food products by Listeria monocytogenes. The present invention also contemplates the use of Listeria monocytogenes bacteriophage, and polynucleotides and polypeptides derived therefrom, for the treatment of host infections or environmental contamination by Listeria monocytogenes.
Owner:INTRALYTIX
Stabilization of fresh mozzarella cheese using fermented whey
InactiveUS7323204B2Improve securityPromote growthMilk preparationDough treatmentBacteroidesMozzarella cheese
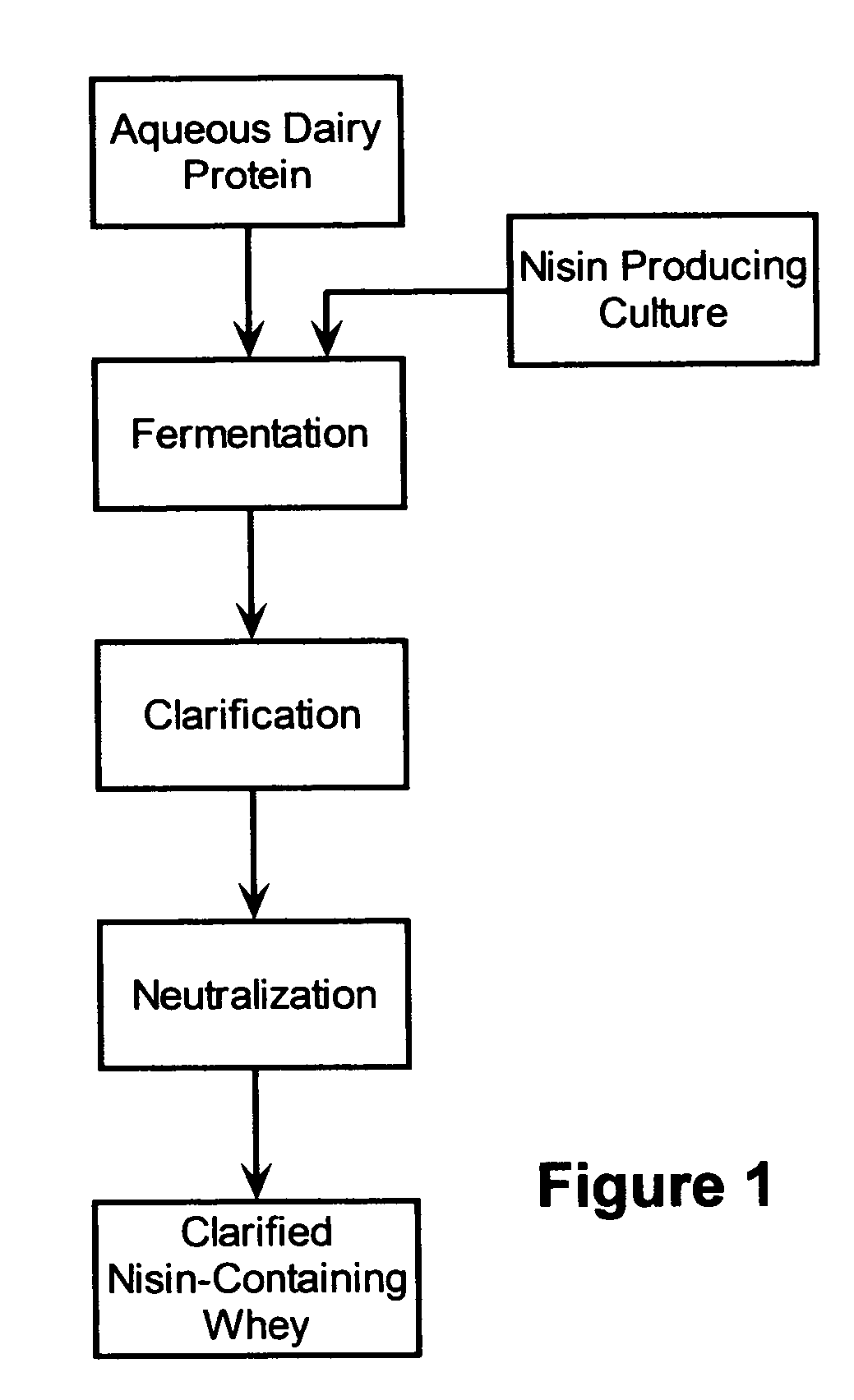
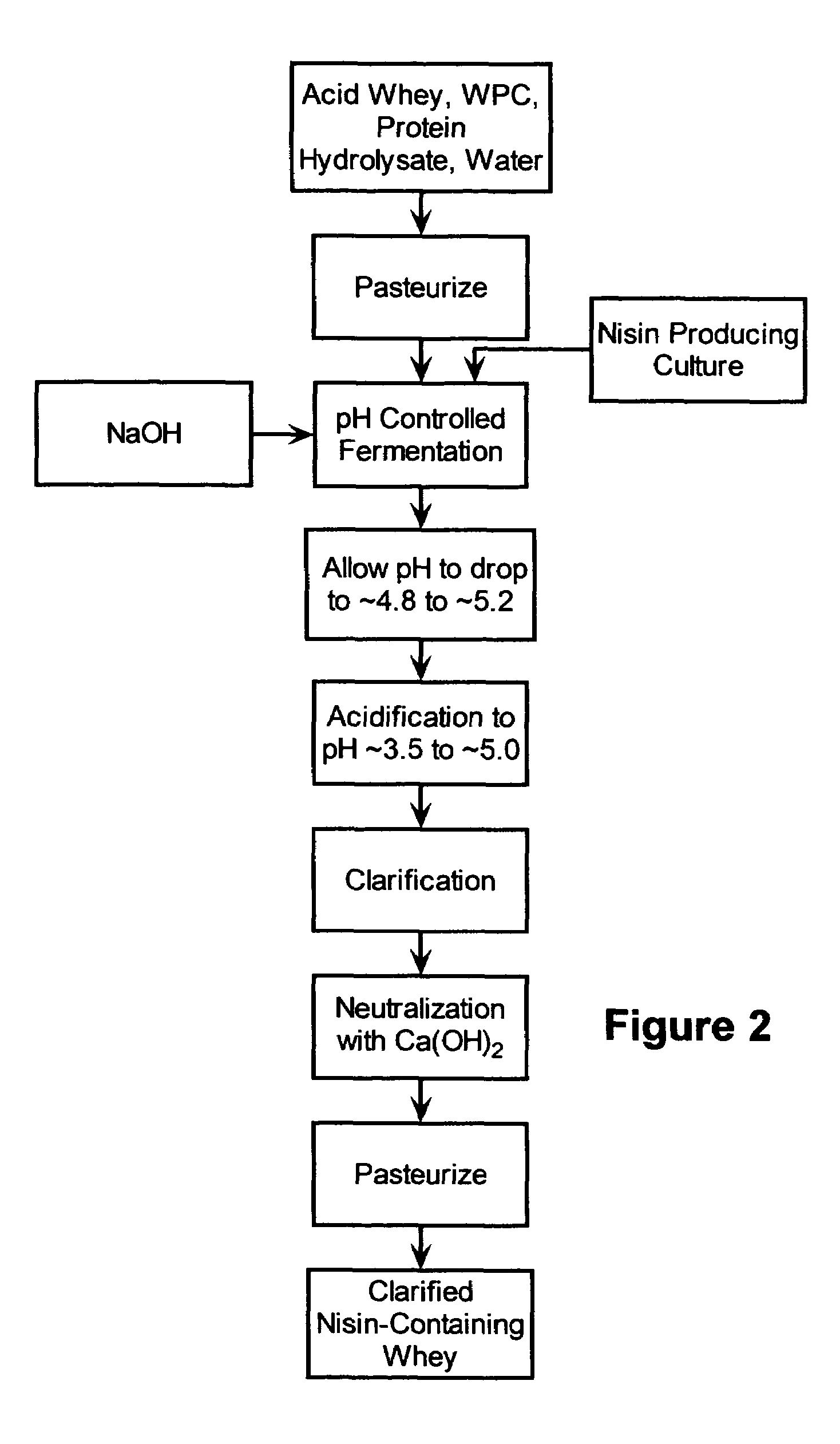
The invention is directed to a fermented and clarified nisin-containing whey and a method of making that can be used to produce a stabilized food product by adding, for example, to the pack water of fresh mozzarella cheese. The resulting stabilized food product retards or limits below detection levels the growth of toxins from pathogenic bacterial contaminants when the nisin-containing whey is added in amounts between about 10 to about 30% to the food product. The stabilized food product improves the safety of the food by retarding the growth of Listeria monocytogenes and improves the shelf life of the product by retarding the growth of gas forming bacteria such as bacteria from the Leuconostoc species.
Owner:KRAFT FOODS GRP BRANDS LLC
Novel listeria monocytogenes bacteriophage and uses thereof
The present invention is directed to isolated Listeria monocytogenes bacteriophage, and methods of using Listeria monocytogenes bacteriophage, or polynucleotides and polypeptides derived therefrom, to control the growth or contamination of food products by Listeria monocytogenes. The present invention also contemplates the use of Listeria monocytogenes bacteriophage, and polynucleotides and polypeptides derived therefrom, for the treatment of host infections or environmental contamination by Listeria monocytogenes.
Owner:INTRALYTIX
Detection of listeria species in food and environmental samples, methods and compositions thereof
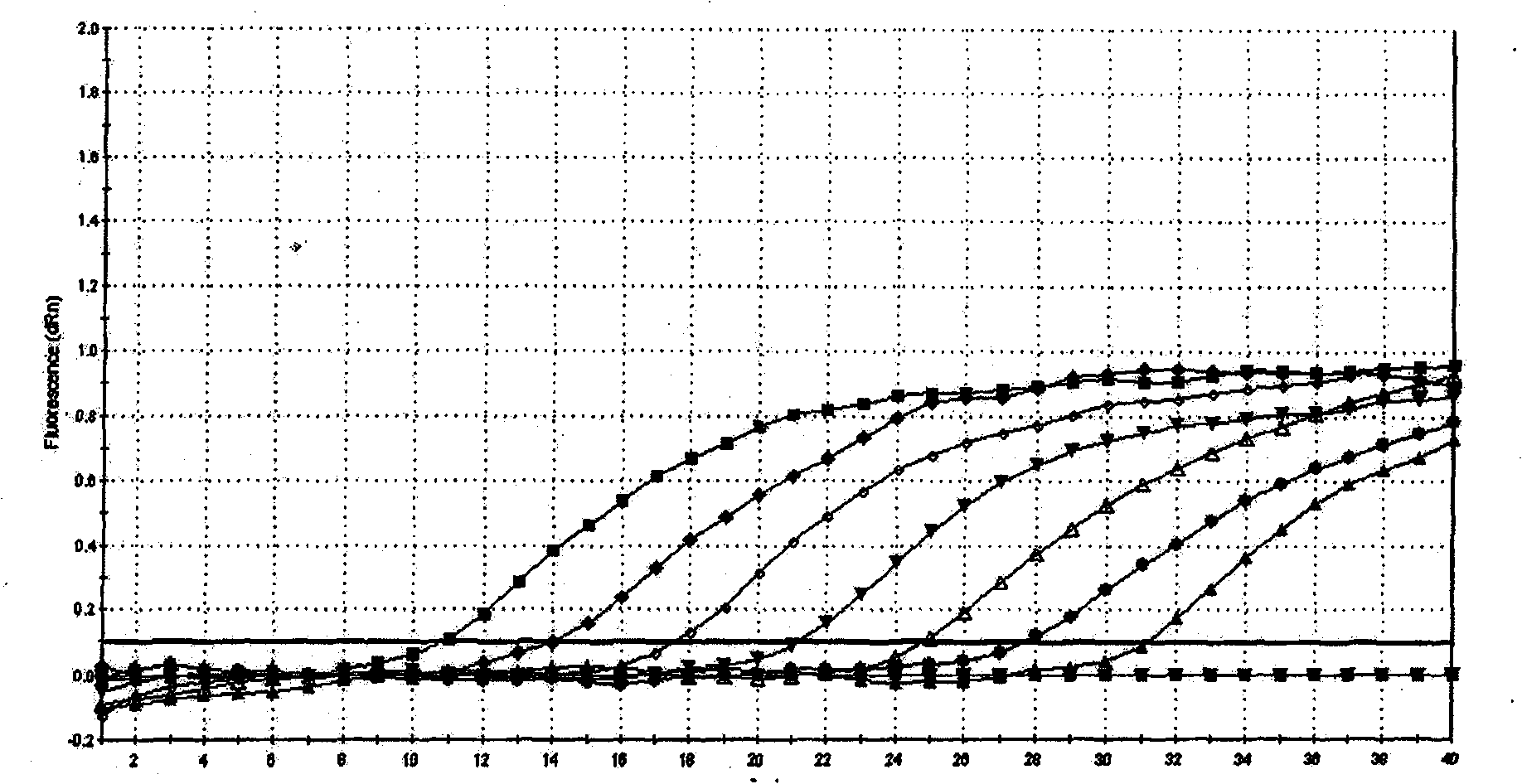
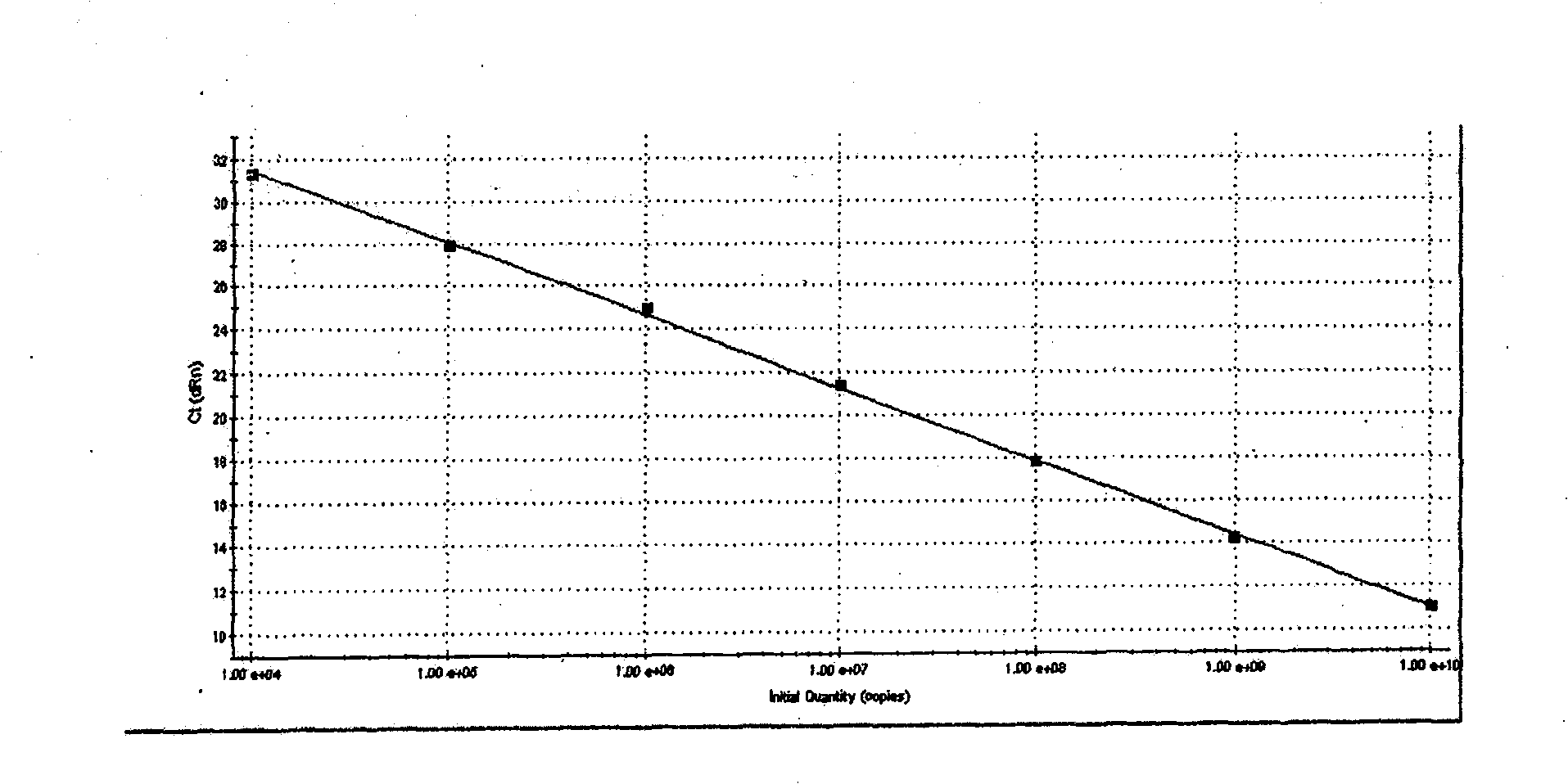
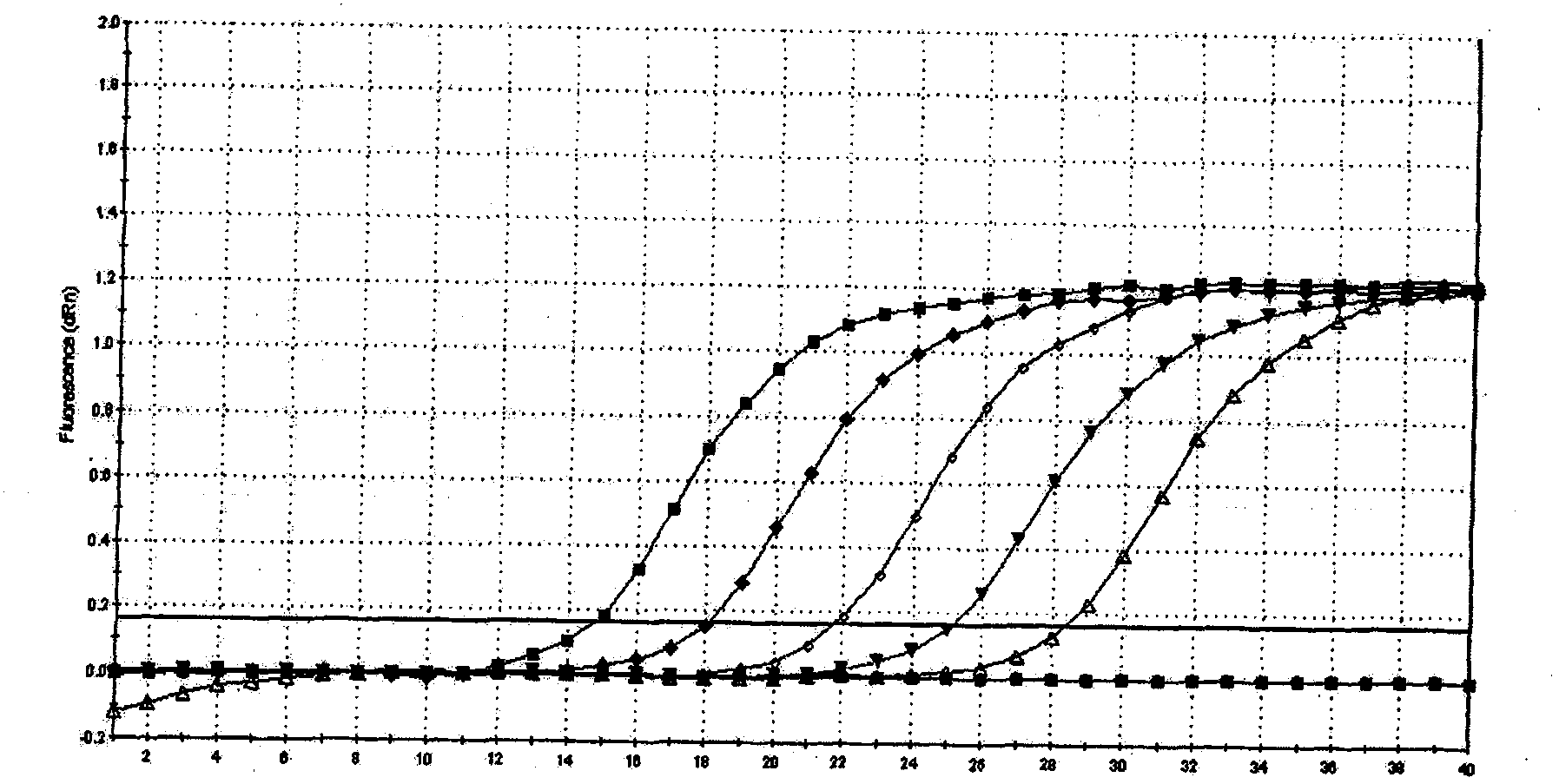
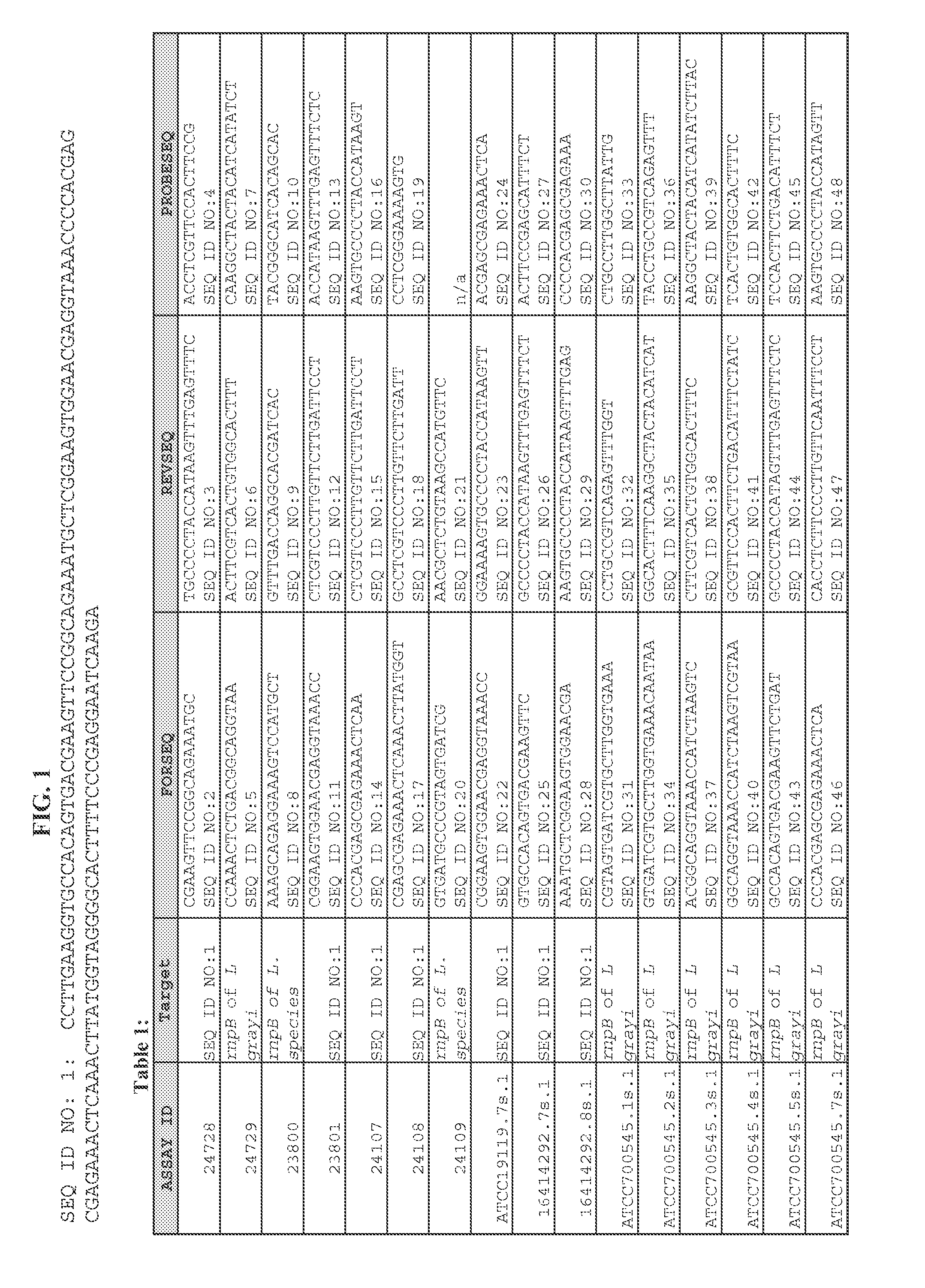
ActiveUS20120009574A1Time-to-result be greatly reducedSensitive detectionSugar derivativesMicrobiological testing/measurementBiotechnologyNucleic acid sequencing
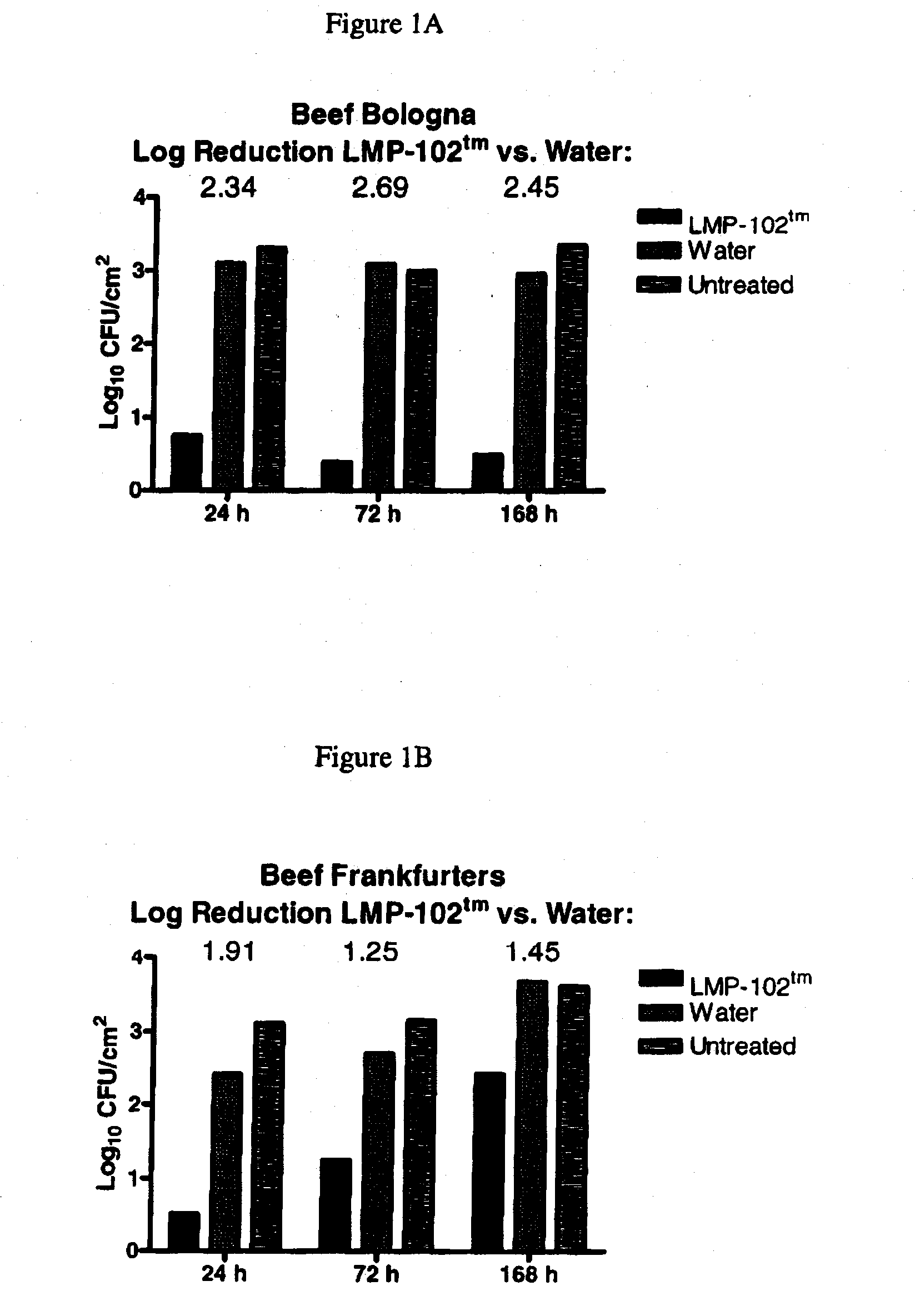
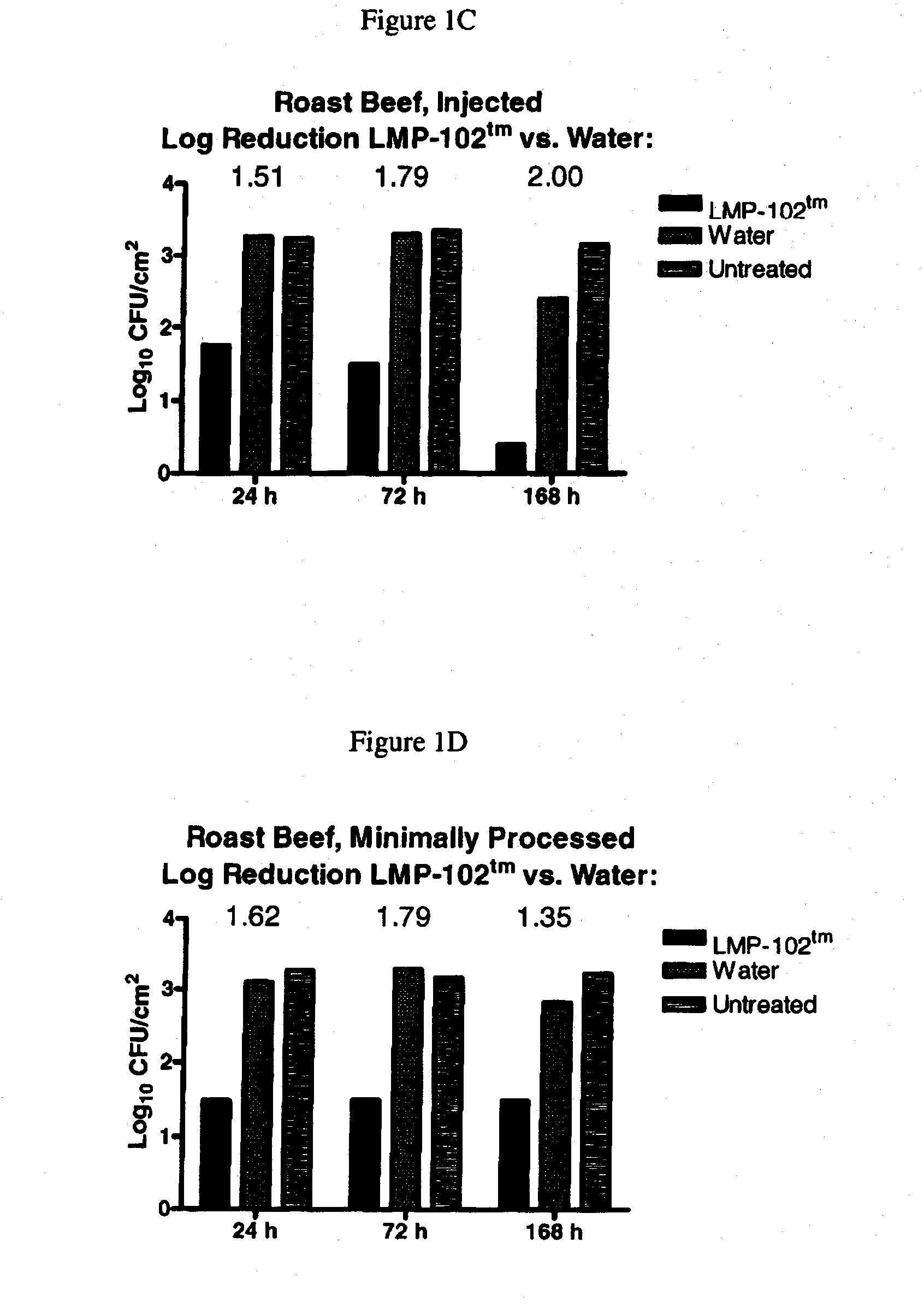
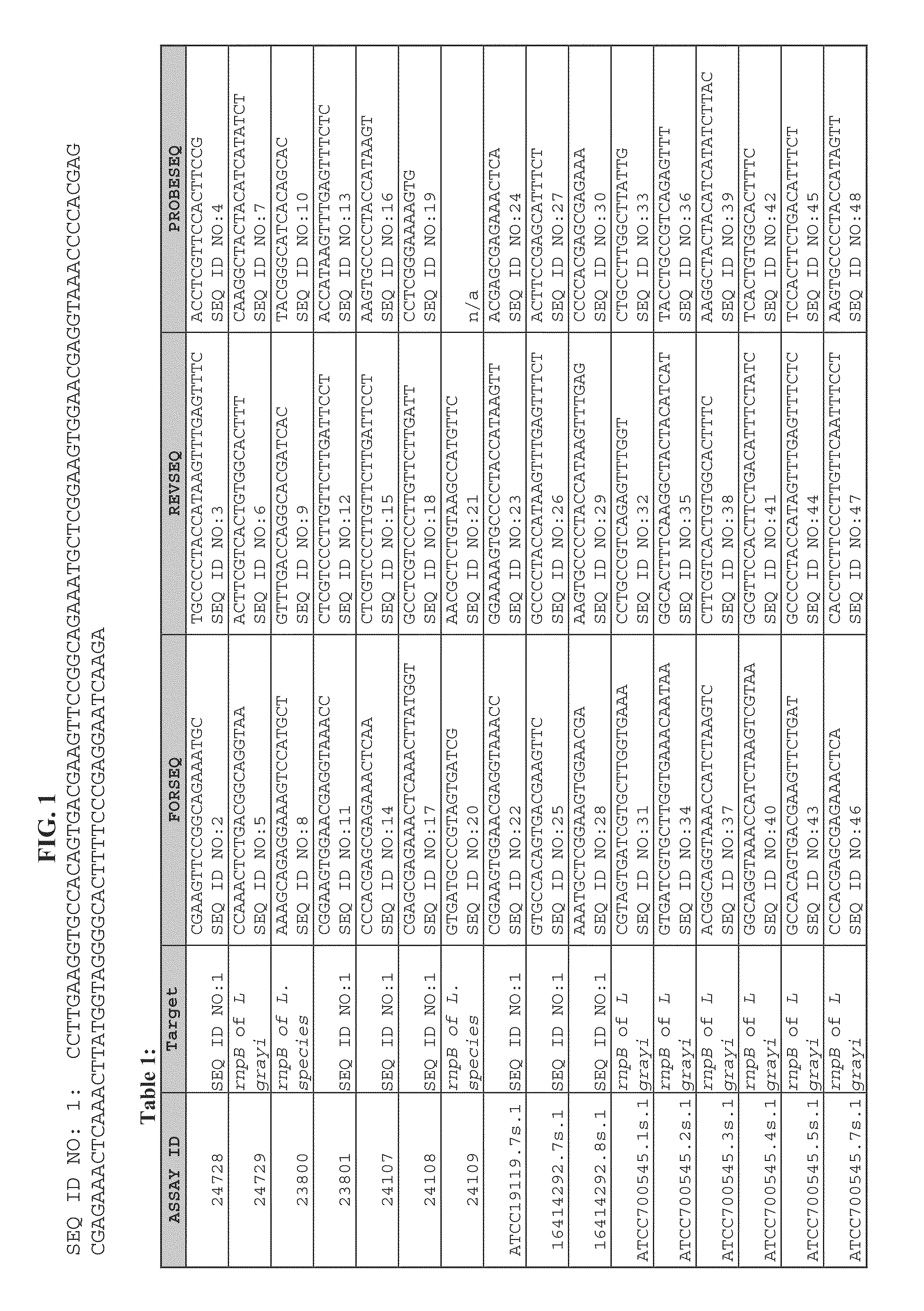
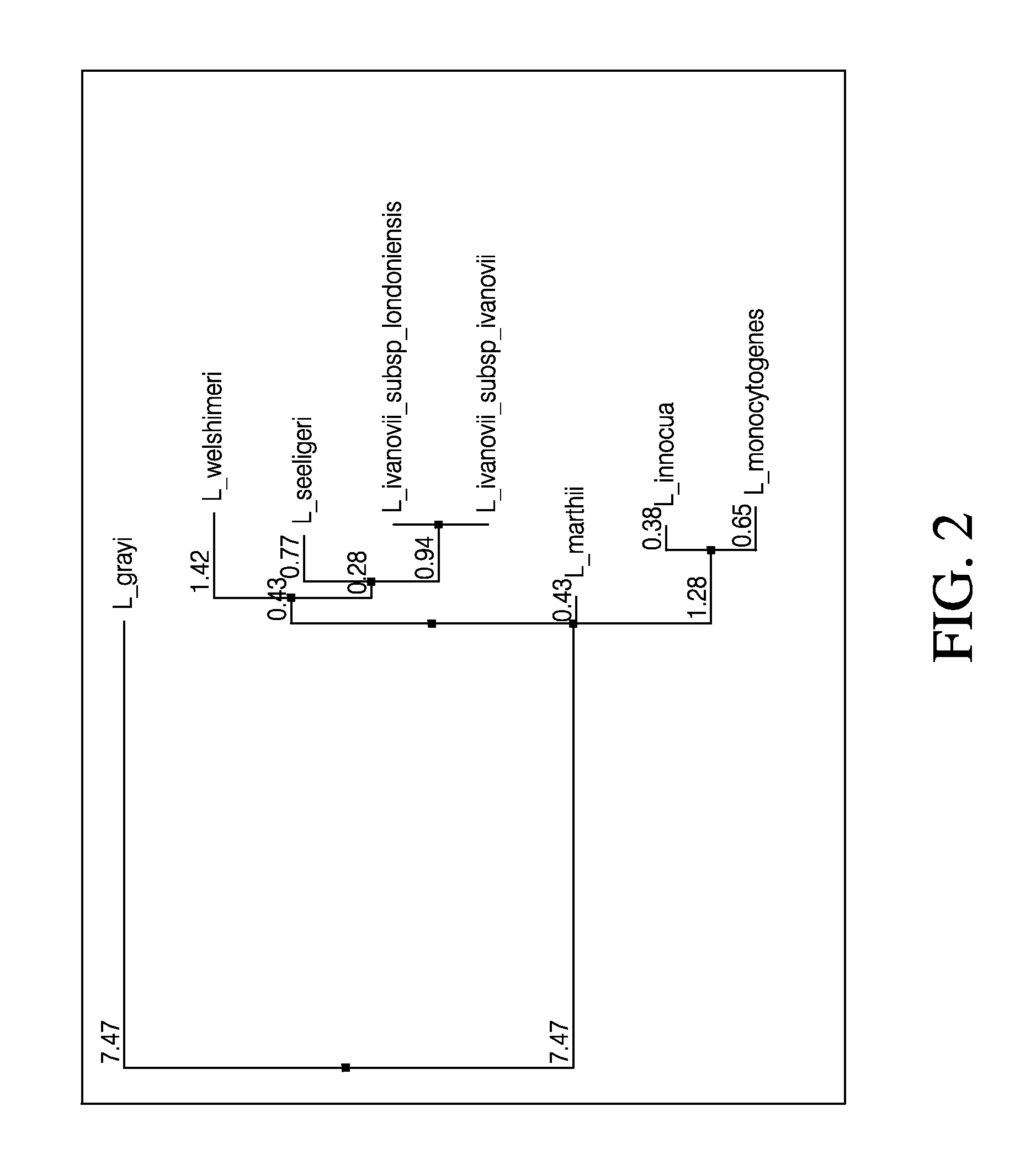
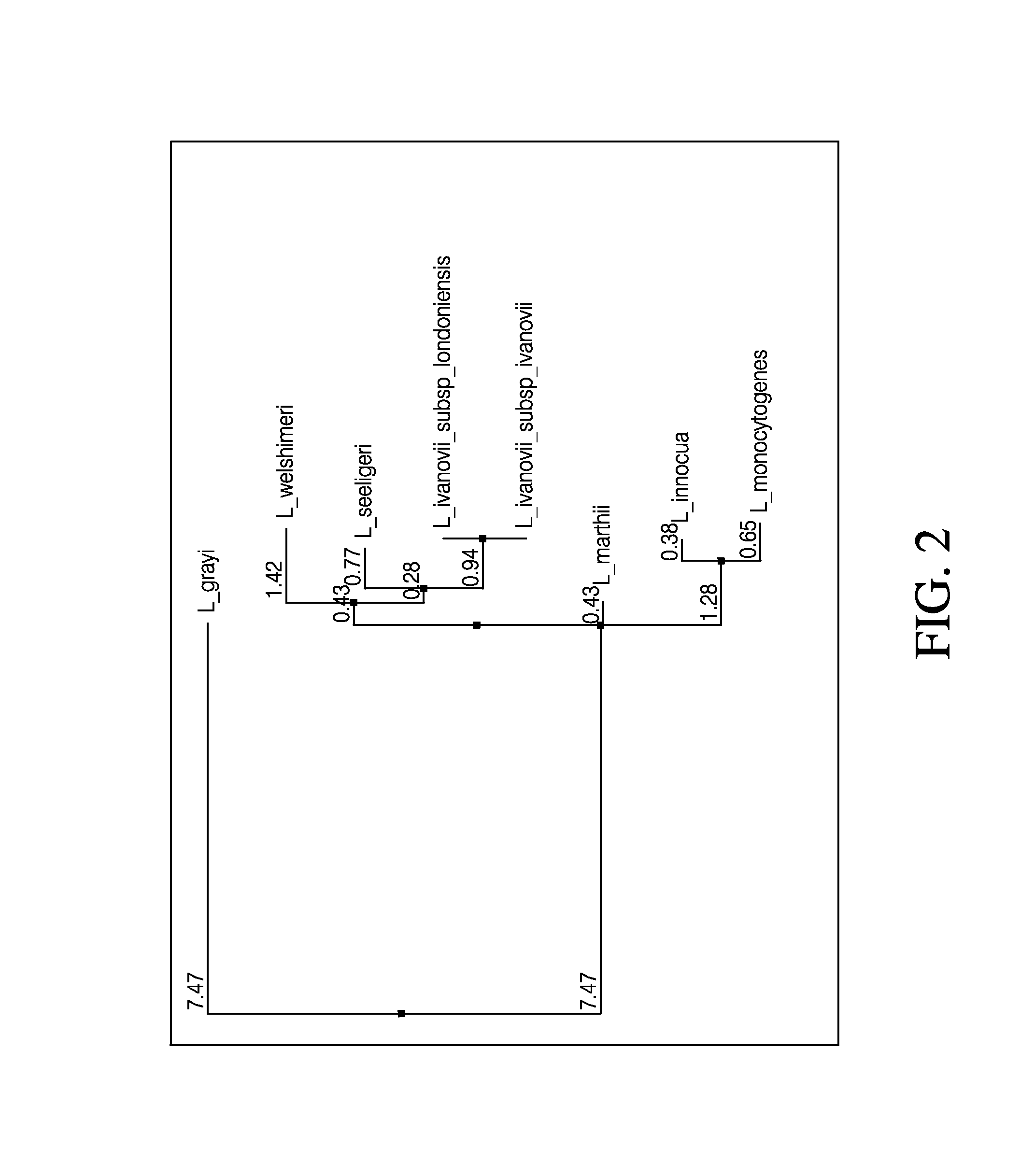
Embodiments of the disclosure relate to isolated nucleic acid sequences, methods of use thereof, and workflows for detection of several Listeria species in a sample, particularly in a food or environmental sample. Embodiments of the disclosure may also be used to detect one or more species or strains of Listeria from each other, for example L. grayi may be detected independently of other Listeria spp. Some embodiments also describe a duplexed assay that can detect L. monocytogenes, L. innocua, L. welshimeri, L. seelgeri, L. marthii (formerly incertae-sedis), L. ivanovii, and L. grayi. Kits for detection of Listeria are also described. In some embodiments, methods and kits of the disclosure may comprise a TAQMAN® assay. In some embodiments, 0.2-2 cfu of Listeria spp. are detected using the compositions, methods and kits after a 24-28 hour enrichment period.
Owner:LIFE TECH CORP
Polynucleotide, method and kit for detecting listeria monocytogenes
ActiveCN105177127AImprove uniformityImprove stabilityMicrobiological testing/measurementDNA preparationFluorescenceDNA construct
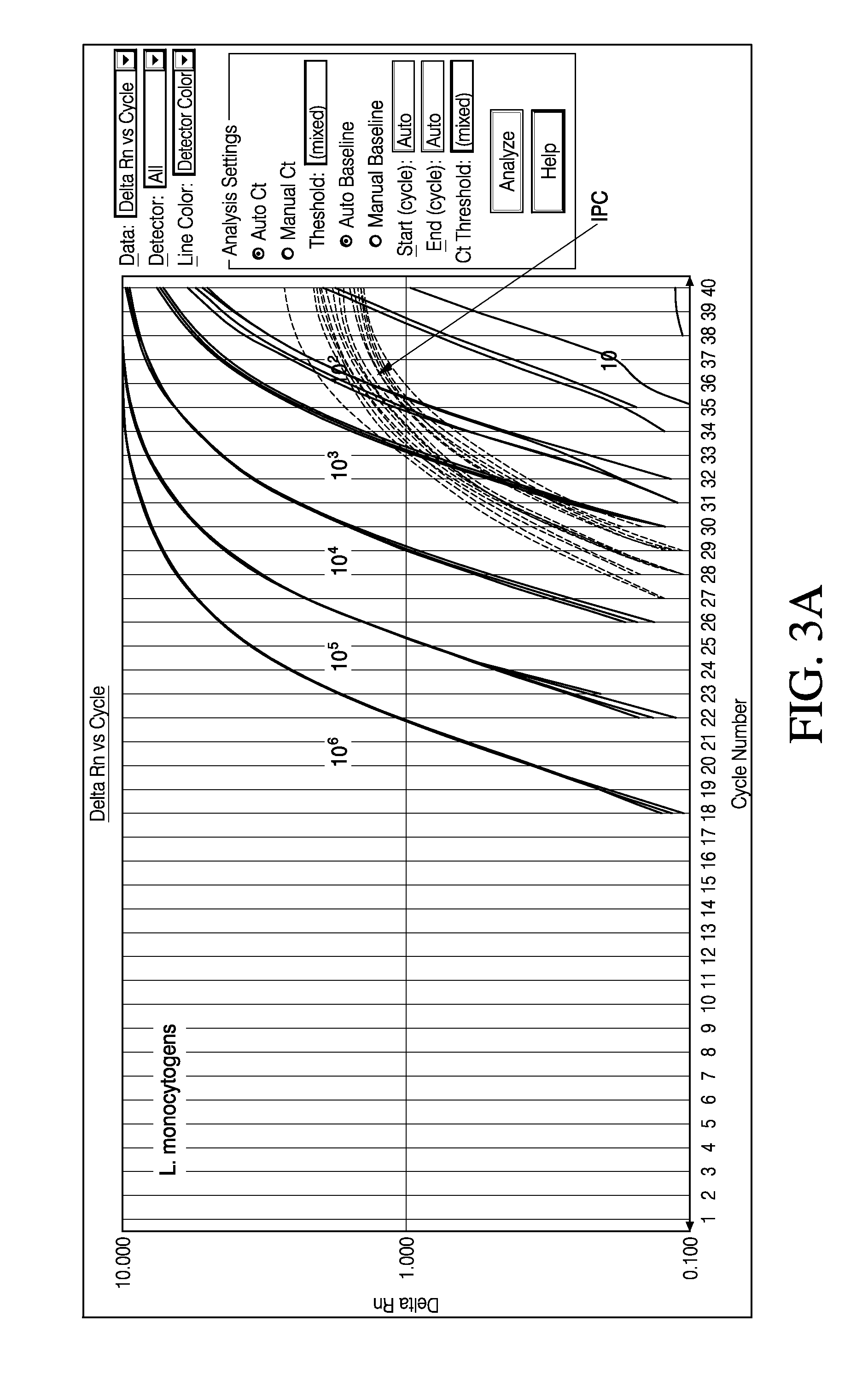
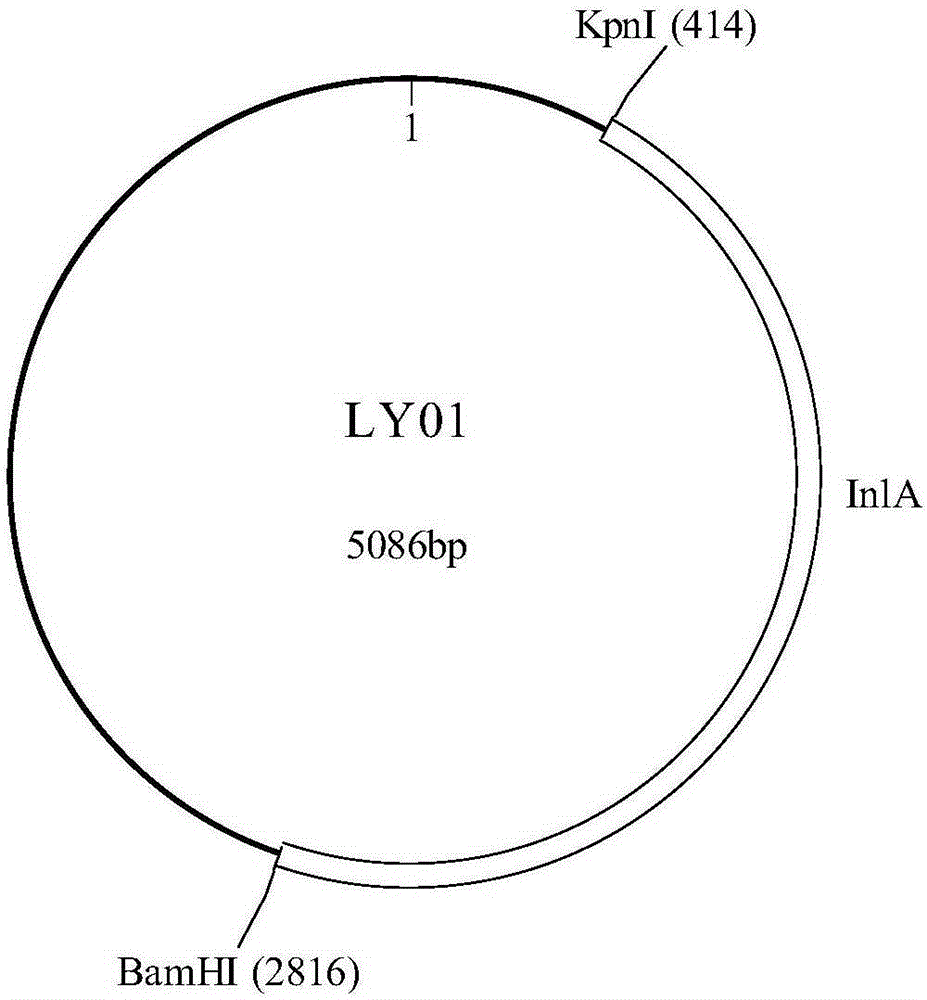
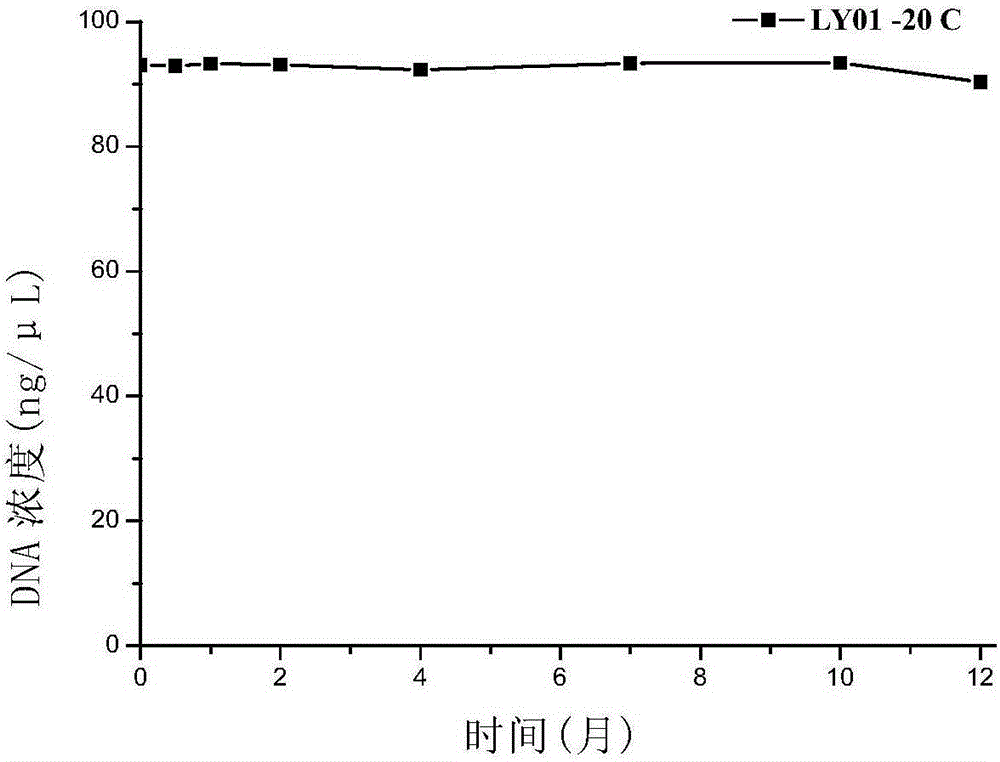
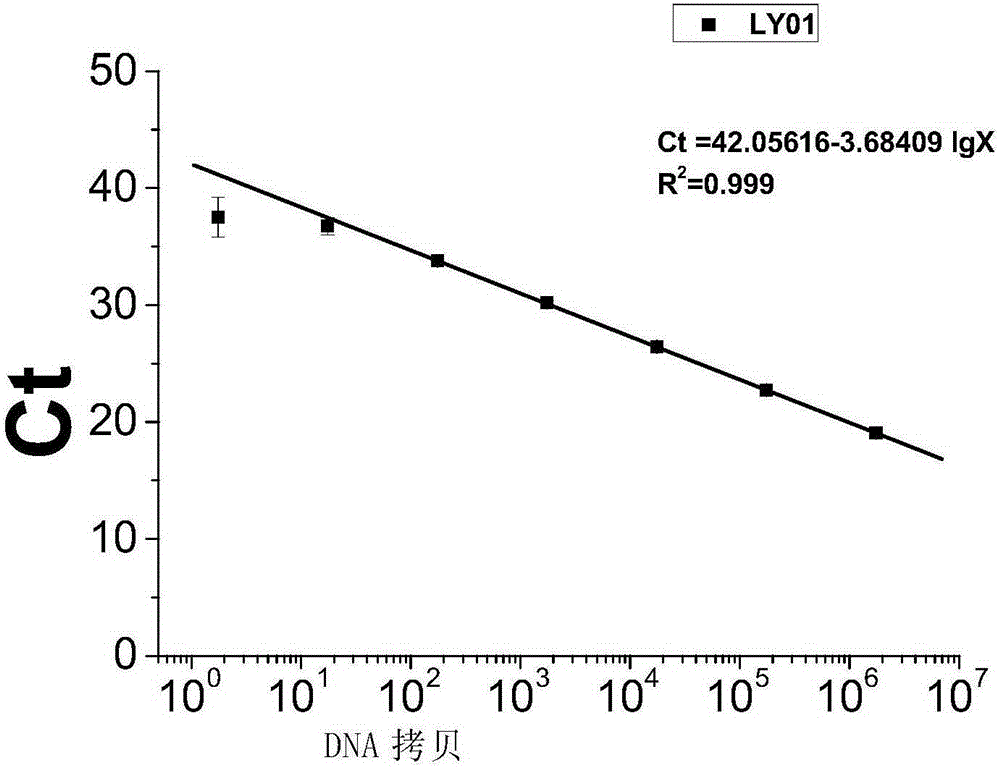
The invention discloses polynucleotide, a method and a kit for detecting listeria monocytogenes. Specifically, the invention discloses polynucleotide, which can be used as a standard molecule in realtime fluorescent PCR detection of listeria monocytogenes, and DNA constructs (LY01 and LY02). The provided plasmid standard molecule can solve the problem that realtime fluorescence PCR detection of listeria monocytogenes is lack of standard substances, and moreover, can guarantee the comparability of the detection results of realtime fluorescence PCR detection. A reliable quality control method is provided for the realtime fluorescence PCR detection of listeria monocytogenes.
Owner:SHANGHAI INST OF MEASUREMENT & TESTING TECH
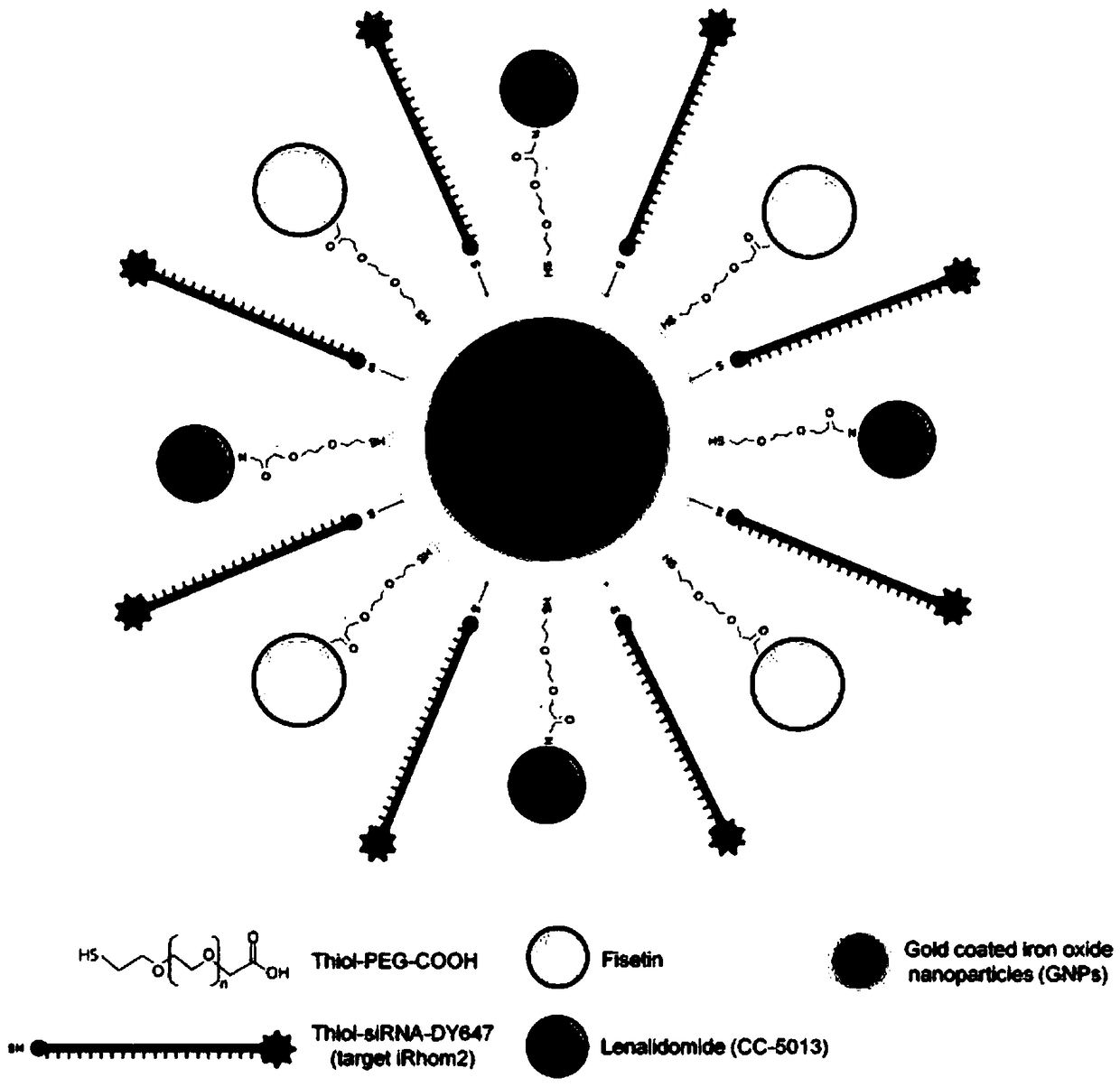
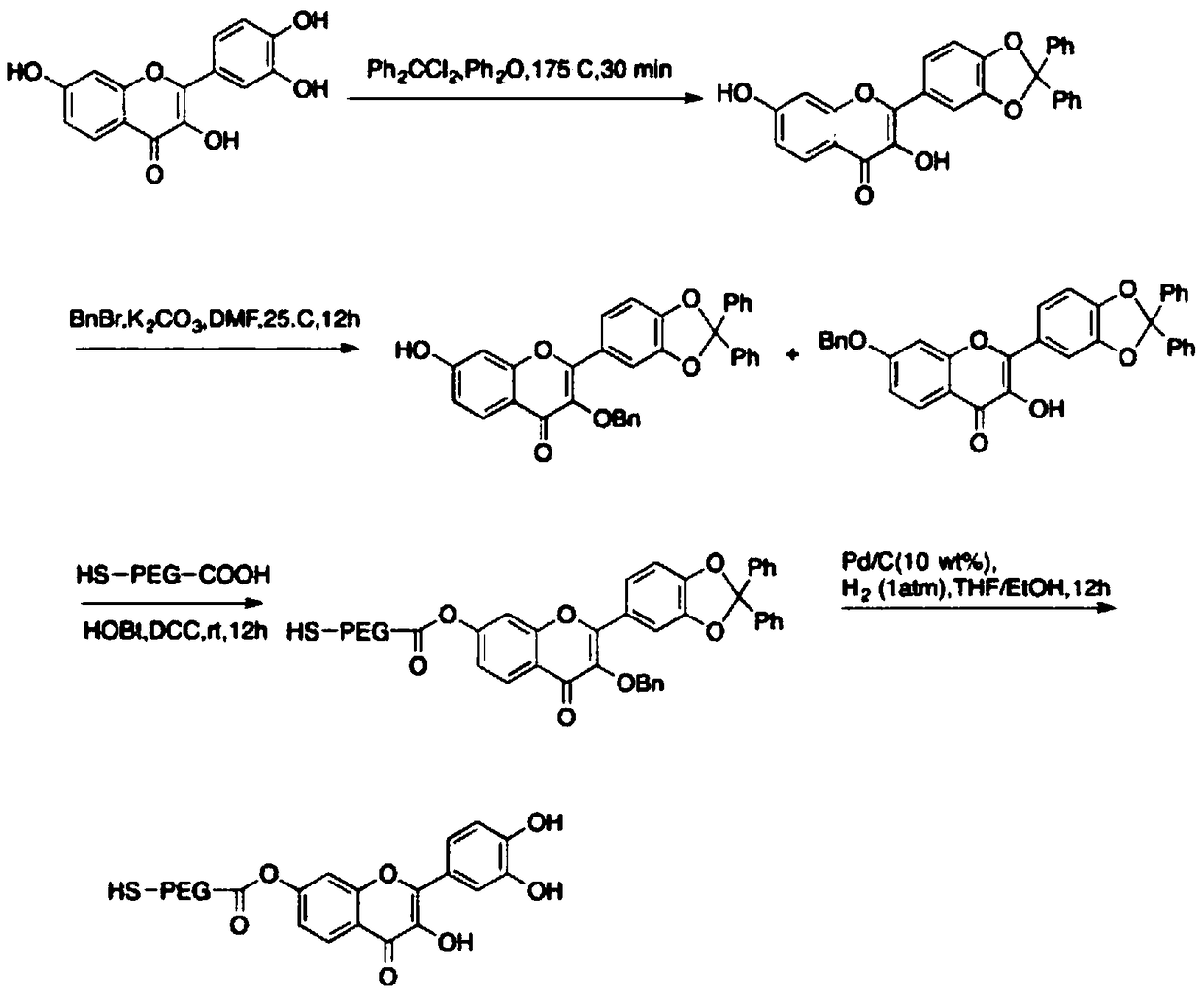
Multi-drug-carrying targeted nanoparticle and preparation method and application thereof
InactiveCN109045056AImprove bioavailabilityEffective acute liver failureAntibacterial agentsOrganic active ingredientsDiseasePolyphenol
The invention belongs to the technical field of biological medicines, and particularly relates to a multi-drug-carrying targeted nanoparticle and a preparation method and application thereof. The multi-drug-carrying targeted nanoparticle is prepared by loading a polyphenol compound, iRhom2 siRNA and TNF-Alpha inhibitor on a gold-coated iron oxide core-shell nanoparticle (GNPs), and the molar ratioof the gold-coated iron oxide core-shell nanoparticle, the polyphenol compound, iRhom2 siRNA and the TNF-Alpha inhibitor is 1:(1 to 3):(1 to 3):(1 to 3). The multi-drug-carrying targeted nanoparticledisclosed by the invention increases the bioactivity and bioavailability of the polyphenol compound, and can effectively prevent and treat related diseases as the result of septicemia caused by listeria monocytogenes.
Owner:CHONGQING UNIV OF EDUCATION
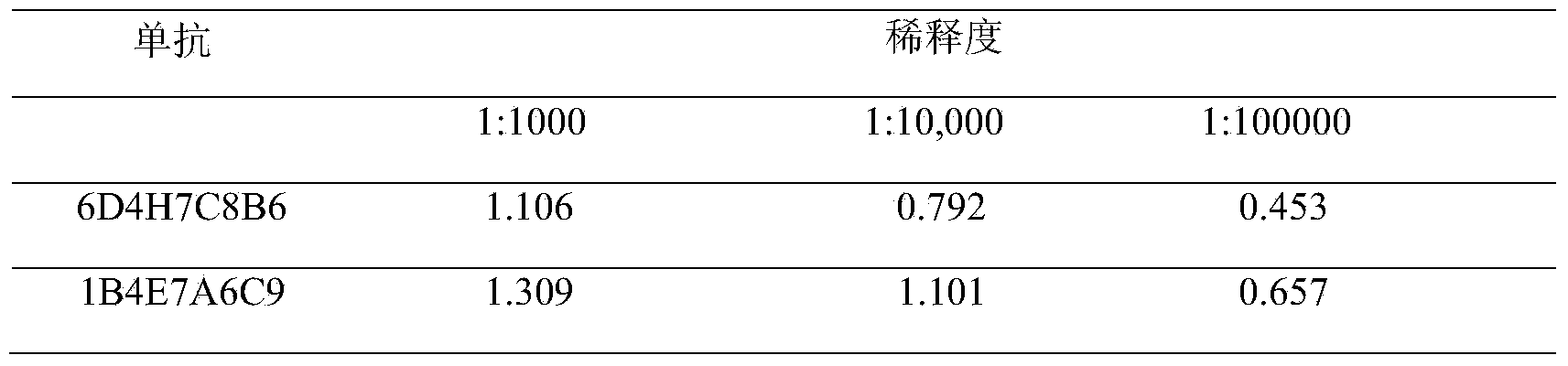
Listeria monocytogenes enzyme-linked immunosorbent assay kit
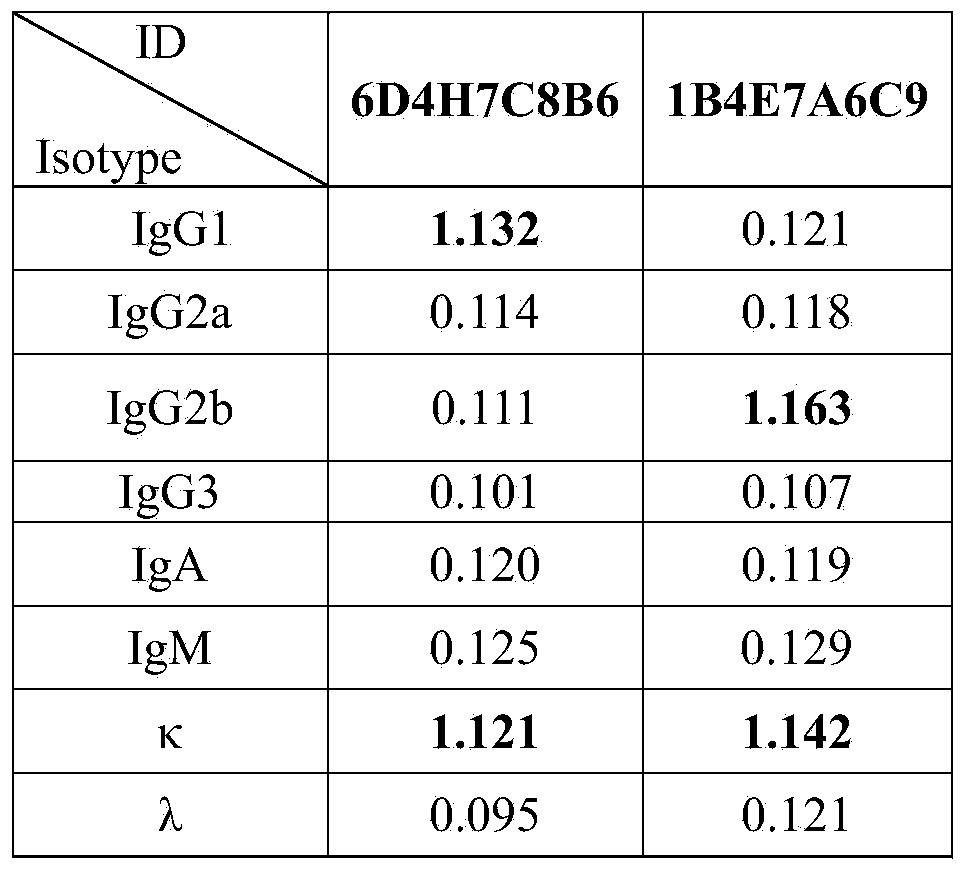
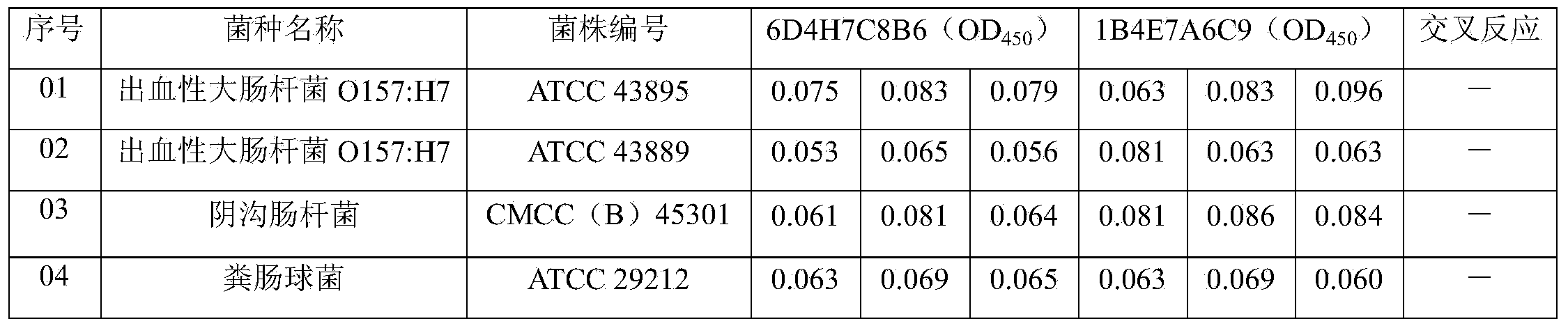
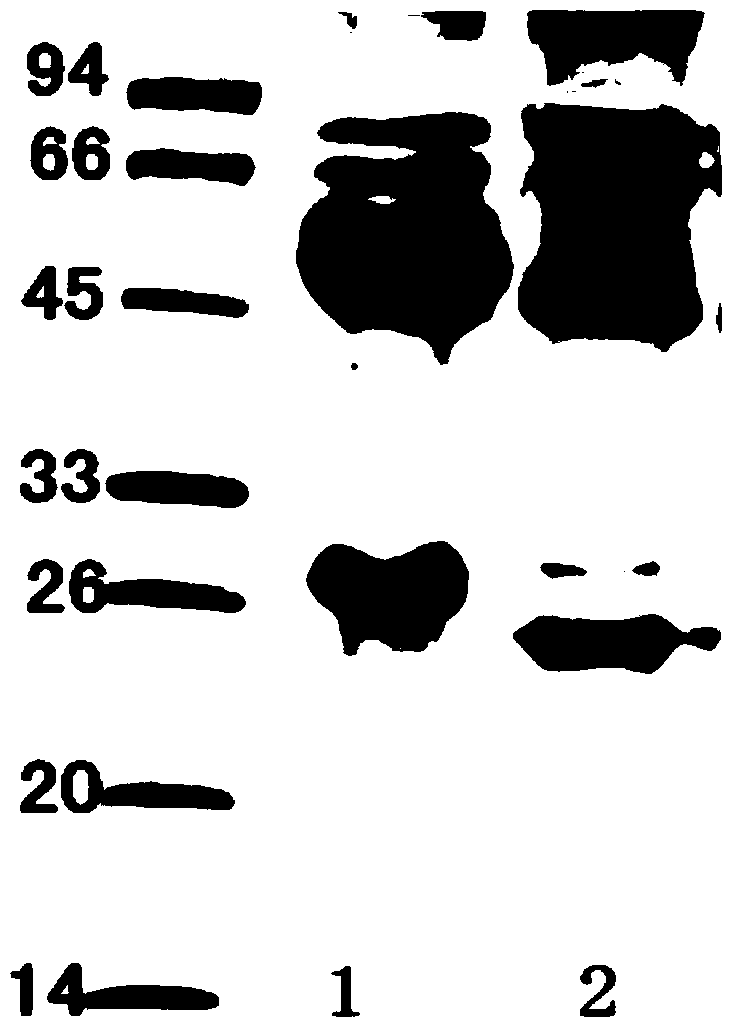
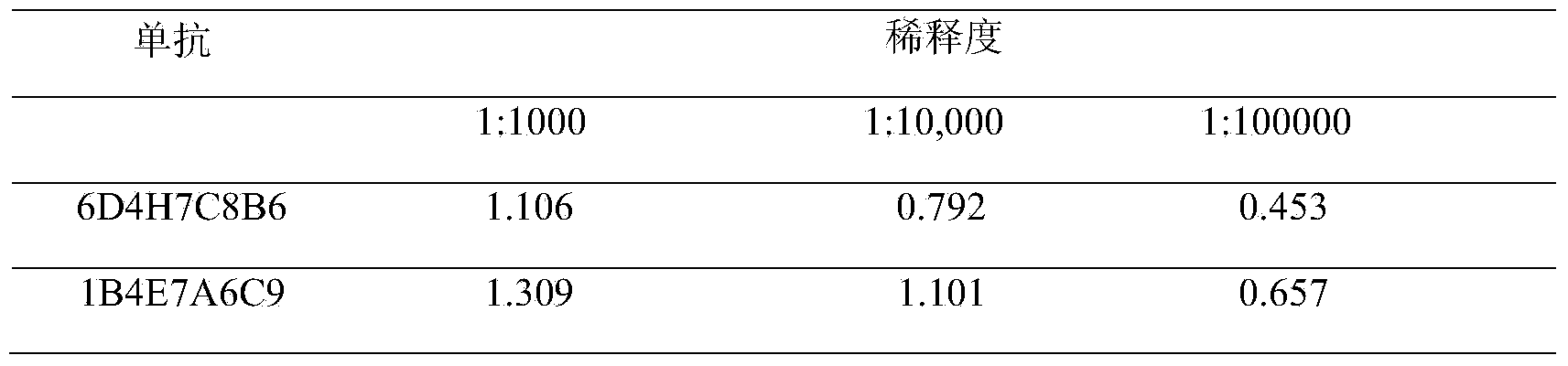
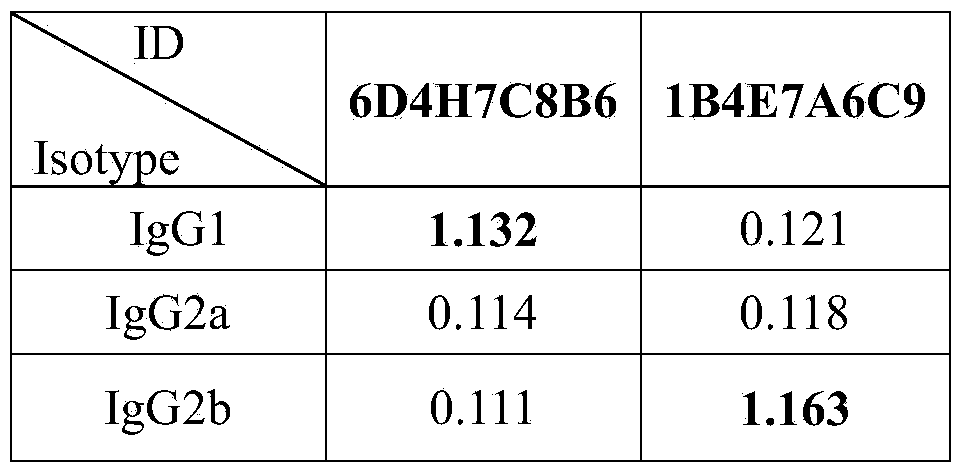
The invention discloses a Listeria monocytogenes enzyme-linked immunosorbent assay kit. The kit contains two monoclonal antibodies which can be specifically bonded to the Listeria monocytogenes, wherein one monoclonal antibody is 1B4E7A6C9 specially used for capturing the antibody, and other monoclonal antibody is 6D4H7C8B6 used for detecting the antibody. The kit is proved to be capable of detecting the Listeria monocytogenes specifically in a high-efficiency manner and having no cross reaction with other 68 common pathogenic bacteria through substantive tests, and therefore, the kit has good performances on detecting the pathogenic bacteria.
Owner:UNIV OF SHANGHAI FOR SCI & TECH
Virulent phages to control Listeria monocytogenes in foodstuffs and in food processing plants
ActiveUS7438901B2Shorten the counting processIncreased substrate specificityAntibacterial agentsBiocideBiotechnologyBacteroides
The present invention relates to virulent (lytic) Listeria monocytogenes phage from the Myoviridae family, preferably P100, alone or in combination with other virulent phages. P100 and the endolysin from P100 can be administered to food products, to the components that will be added to food products, and / or to the infrastructure of the food processing plants within which such food products are processed, or the containers or wraps in which such foods are stored and / or shipped, in order to reduce Listeria monocytogenes contamination. P100 can also be used in the present invention to identify Listeria monocytogenes bacteria present on (or within) foodstuffs, as well as those Listeria monocytogenes bacteria present in the equipment or the general environment of the food processing plants in which the foodstuffs are being processed and in animals infected with Listeria monocytogenes. The phage and the endolysin of the present invention can also be used to treat animals infected with Listeria monocytogenes. P100 will kill the bacteria that are within its host range with great efficiency and will propagate to high titer thereon. P100 can be combined with other lytic phage, and / or with other antimicrobial agents to reduce or eliminate Listeria.
Owner:EBI FOOD SAFETY
Method for detecting Listeria monocytogenes and monoclonal antibodies
ActiveCN103941011AImmunoglobulins against bacteriaMicroorganism based processesMicroorganismMonocyte
The invention belongs to the field of microbiological detection and in particular relates to a method for detecting Listeria monocytogenes and two Listeria monocytogenes resisting monoclonal antibodies generated in the same monoclonal antibody preparation process for the method. The monoclonal antibodies can be specifically bonded with the Listeria monocytogenes and is generated by mouse hybridoma cell line 6D4H7C8B6, CGMCC No.8002 or 1B4E7A7C9, CGMCC No.8765. The invention also provides application of the Listeria monocytogenes resisting monoclonal antibodies.
Owner:UNIV OF SHANGHAI FOR SCI & TECH
Multiple PCR detection kit for Listeria monocytogenes and Listeria illichii
ActiveCN110184367AStrong specificityHigh sensitivityMicrobiological testing/measurementMicroorganism based processesListeria murrayiMicrobiology
The invention provides a multiple PCR detection kit for Listeria monocytogenes and Listeria illichii. The kit comprises a gene lmo0601 detection primer and a gene smcl detection primer. The inventionestablishes a multiple PCR method for rapidly detecting Listeria monocytogenes and Listeria illichii by using the two pairs of primers, and the method has the advantages of good specificity, high sensitivity, high detection speed, easy operation and simple result judgment.
Owner:YANGZHOU UNIV
Isothermal amplification method for rapid detection of listeria monocytogenes
InactiveCN104593516AImprove responseShorten detection timeMicrobiological testing/measurementMicroorganism based processesGenomic DNAHelicase
The invention provides a real-time fluorescent quantitative helicase-dependent isothermal nucleic acid amplification method for detecting listeria monocytogenes. Primer pair sequences having specific amplification function on listeria monocytogenes are designed so as to reflect a natural process for simulating the replication of in-vivo DNA at a constant temperature, DNA double strands are solved at a constant temperature by virtue of helicase, the listeria monocytogenes genomic DNA is adopted as a template, specific isothermal nucleic acid amplification is performed so as to achieve the exponential growth of the target sequence, the real-time fluorescent helicase-dependent isothermal nucleic acid amplification detection method is established, detection results are analyzed by virtue of an isothermal amplification curve and melting curve of the genomic DNA so that listeria monocytogenes is rapidly detected. By the method, the disadvantage that the traditional PCR needs to rely on the repeated heating and cooling of an instrument to obtain the single-stranded template is overcome and the method is simple in principle, convenient to operate and listeria monocytogenes in foods can be rapidly, simply, sensitively and specifically detected.
Owner:JIANGNAN UNIV
Method for enriching and separating listeria monocytogenes
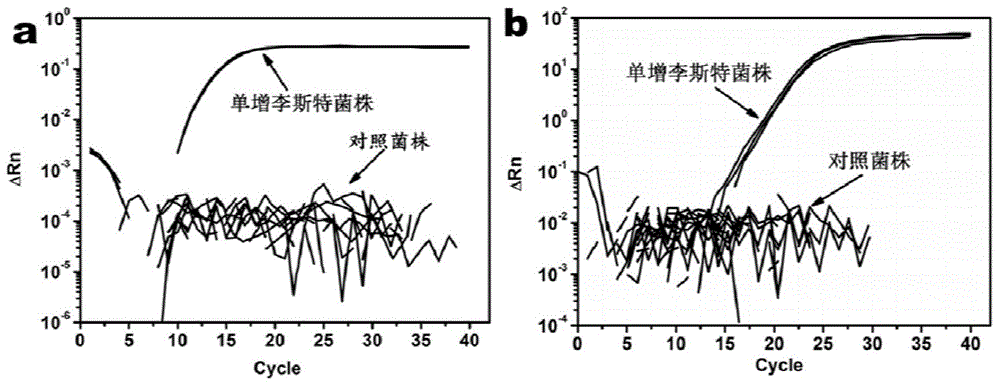
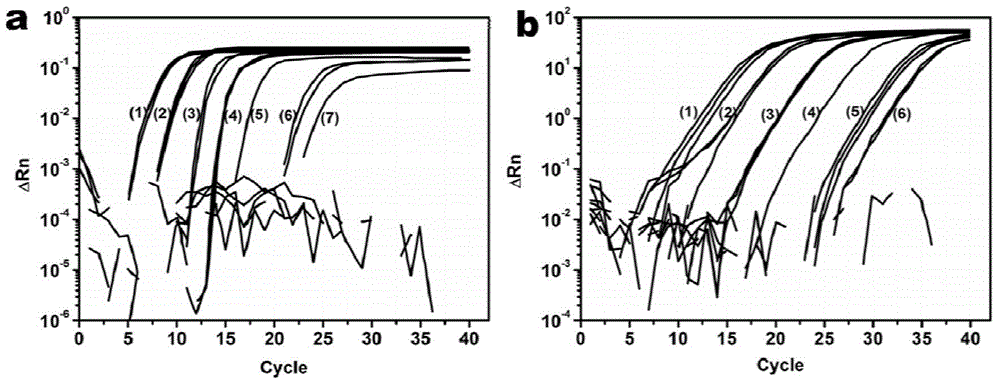
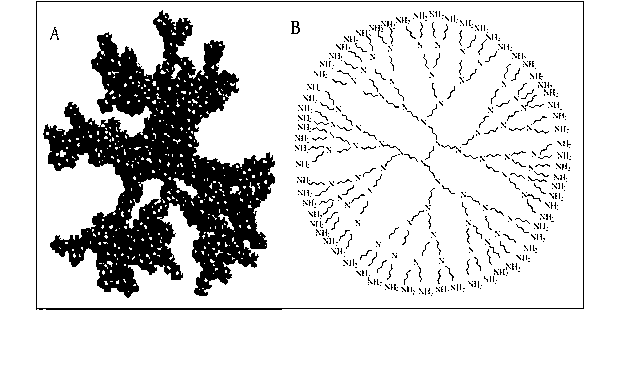
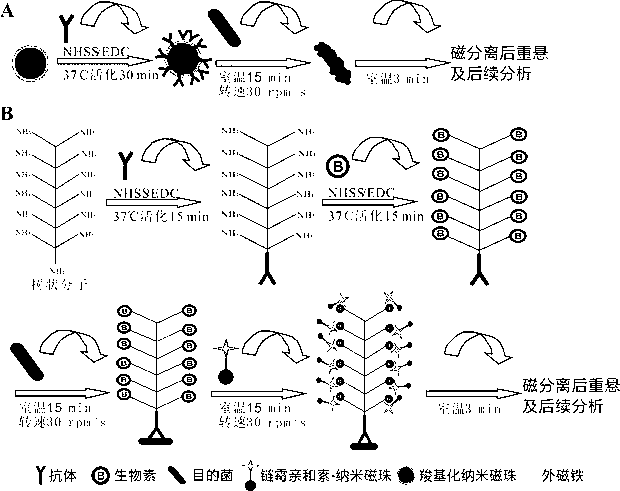
InactiveCN103275903AAchieve separationEasy to separateBacteriaMicroorganism based processesDendrimerBacteroides
The invention discloses a method for enriching and separating listeria monocytogenes (Listeria monocytogenes, Lm), provides a basis for subsequent research on target bacteria and relates to the technical field of biology. The method comprises the following steps: performing covalent coupling on a dendrimer and an antibody, coating a long-chain biotin molecule on the antibody-modified dendrimer, capturing the target bacteria in a sample solution through the dendrimer modified by the antibody and the long-chain biotin, identifying streptavidin-modified nano magnetic beads and coupling the long-chain biotin dendrimer in the sample solution, separating and suspending the captured bacteria, wherein suspension can be directly analyzed later. Compared with the traditional bacteria magnetic separation method, the method is suitable for performing magnetic separation on the bacteria in a complex substrate, and the separation efficiency of target bacteria in the sample is improved.
Owner:NANCHANG UNIV
Fluorescent quantitative PCR (polymerase chain reaction) reagent kit for detecting Listeria monocytogenes
InactiveCN102586413AMicrobiological testing/measurementMicroorganism based processesDNA fragmentationMicrobiology
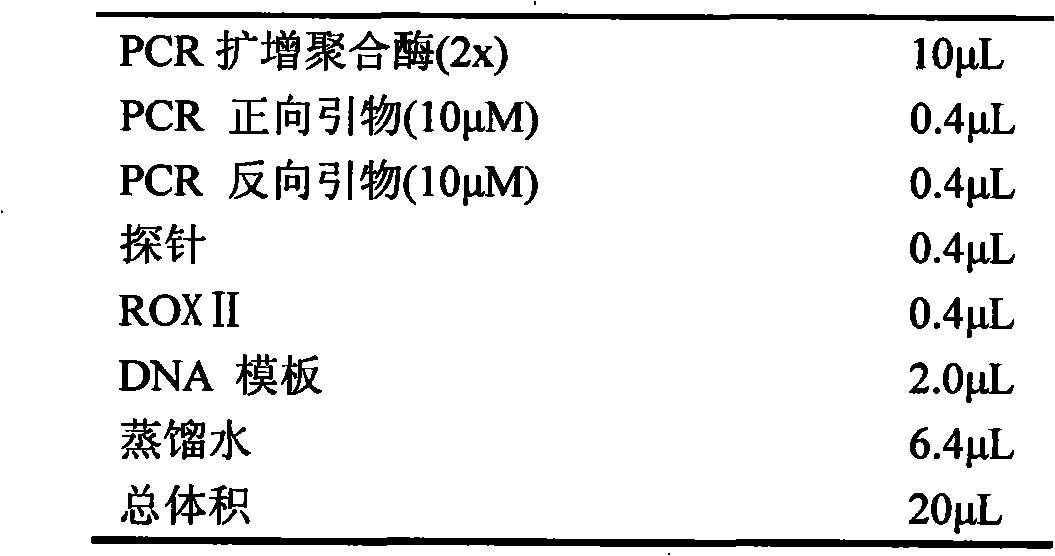
The invention provides a fluorescent quantitative PCR (polymerase chain reaction) reagent kit for detecting Listeria monocytogenes, which comprises a pair of primers, a probe and a positive quantitative template. The sequences of the primers and the sequence of the probe are respectively SEQ ID No.1, SEQ ID No.2 and SEQ ID No.3, the positive quantitative template is pMD 18-T recombinant plasmid with the sequence of SEQ ID No.4, the reagent kit further can comprise fluorescent quantitative polymerase and reference dye, and application of the reagent kit to detecting the Listeria monocytogenes in clinical samples is also provided. Experimental results show that the fluorescent quantitative PCR reagent kit for detecting the Listeria monocytogenes is high in specificity and sensitivity, simple in composition and convenient in operation, and can be used for quickly detecting the Listeria monocytogenes.
Owner:QILU UNIV OF TECH
Detection of Listeria species in food and environmental samples, methods and compositions thereof
ActiveUS8795969B2Sensitive detectionSugar derivativesMicrobiological testing/measurementBiotechnologyNucleic acid sequencing
Embodiments of the disclosure relate to isolated nucleic acid sequences, methods of use thereof, and workflows for detection of several Listeria species in a sample, particularly in a food or environmental sample. Embodiments of the disclosure may also be used to detect one or more species or strains of Listeria from each other, for example L. grayi may be detected independently of other Listeria spp. Some embodiments also describe a duplexed assay that can detect L. monocytogenes, L. innocua, L. welshimeri, L. seelgeri, L. marthii (formerly incertae-sedis), L. ivanovii, and L. grayi. Kits for detection of Listeria are also described. In some embodiments, methods and kits of the disclosure may comprise a TAQMAN® assay. In some embodiments, 0.2-2 cfu of Listeria spp. are detected using the compositions, methods and kits after a 24-28 hour enrichment period.
Owner:LIFE TECH CORP
Adp-ribosylating toxin from listeria monocytogenes
InactiveUS20090297542A1Improve stabilityReduce and eliminate ADP-ribosylating activity of proteinOrganic active ingredientsGenetic material ingredientsBacillus perfringensRibose
An ADP-ribosylating toxin from Listeria monocytogenes is disclosed, together with mutant toxins and uses therefor. There is only a low level of sequence identity between this toxin and known toxins such as the iota toxin from Clostridium perfringens.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Method using liquid phase chip to detect mononuclear cell proliferation listeria
A method using a liquid phase chip to detect mononuclear cell proliferation listeria belongs to the field of immunity technology and food sanitation detection technology. The invention aims to provide the method using the liquid phase chip to detect mononuclear cell proliferation listeria, so as to realize rapid detection of mononuclear cell proliferation listeria. In the invention, the liquid phase chip is prepared and then applied to detect mononuclear cell proliferation listeria. Compared with other detection methods, the method using the liquid phase chip to detect mononuclear cell proliferation listeria has advantage of high two-way throughput of detection sample amount and detection items (a large amount of samples can be detected in items as many as 96), and has the characteristics of good flexibility, high sensitivity, good SNR (signal to noise ratio), convenience for operation, wide standard curve range, wide applicable range and the like. The liquid phase chip technology, as an advanced service platform of bioinformatics, has wide applicable prospect in fields like clinical diagnosis, veterinarian communicable disease diagnosis, food microorganism detection and so on.
Owner:吉林出入境检验检疫局检验检疫技术中心
Primer for detecting Listern nucleotide segment of monocellular hyperplasia and probe sequence
The present invention is PCR proliferation primer and probe sequence for detecting nucleotide segment of monocellular hyperplasia Listeria. The primer sequence includes the primer pair comprising upstream primer sequence LMF1420 of CTGAATCTCAAGCAAAACCTGGT and downstream primer sequence LMR1593 of CGCGACCGAAGCCAACTA, as well as 10 bases extending in 5' end direction and 10 bases extending in 3' end direction of upstream primer LMF1420 and 9 bases extending in 3' end direction and 5 bases extending in 5' end direction of downstream primer LMR1593. The probe sequence includes probe LM-p1 sequence ATACGATAACATCCACGGCTCTGGCTGG, 9 bases extending in the 3' end direction and 10 bases extending in the 5' end direction.
Owner:TAITAI GENOMICS
Immune PCR (polymerase chain reaction) detection kit for Listeria monocytogenes
ActiveCN103937898AMicrobiological testing/measurementDNA/RNA fragmentationImmuno pcrMonoclonal antibody
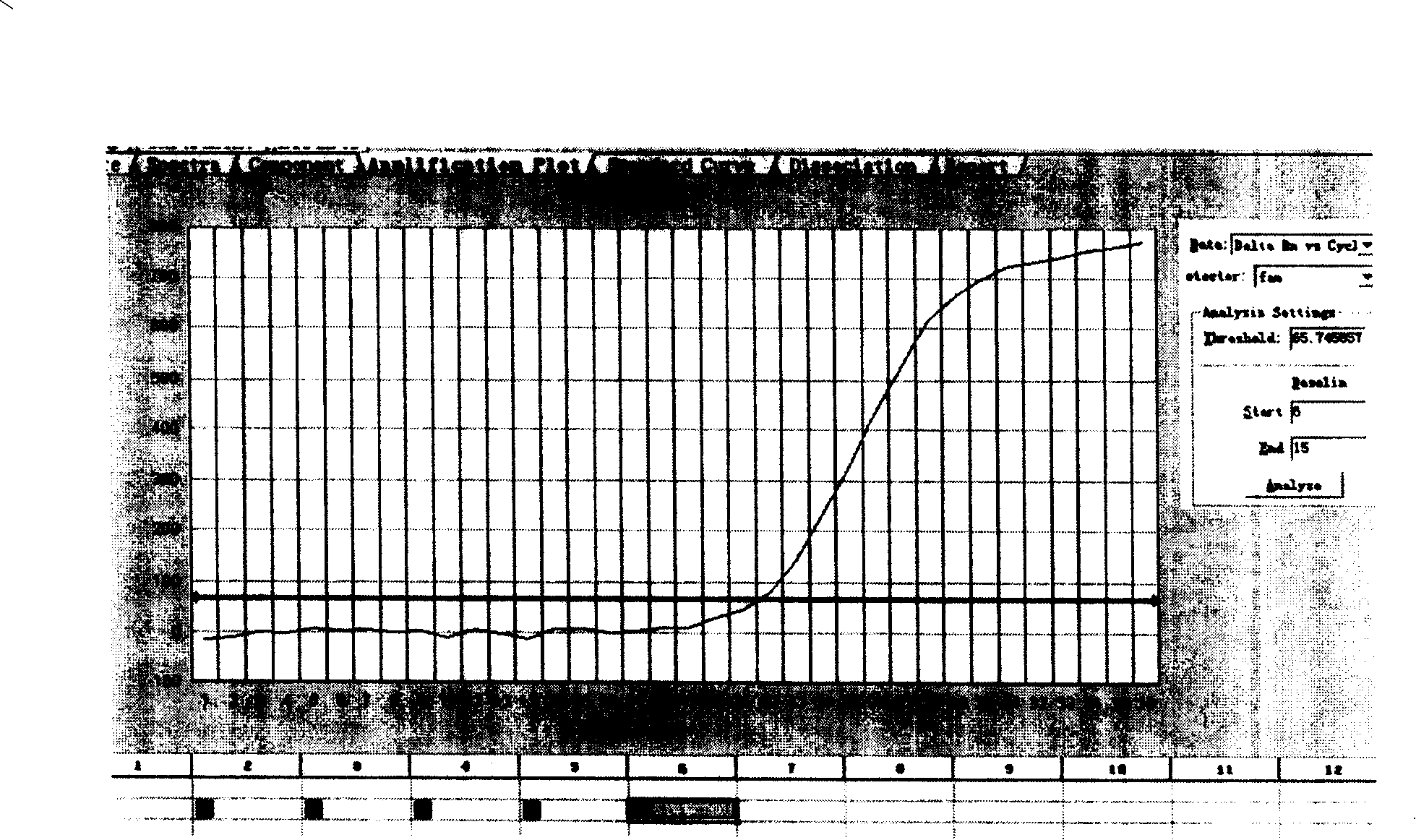
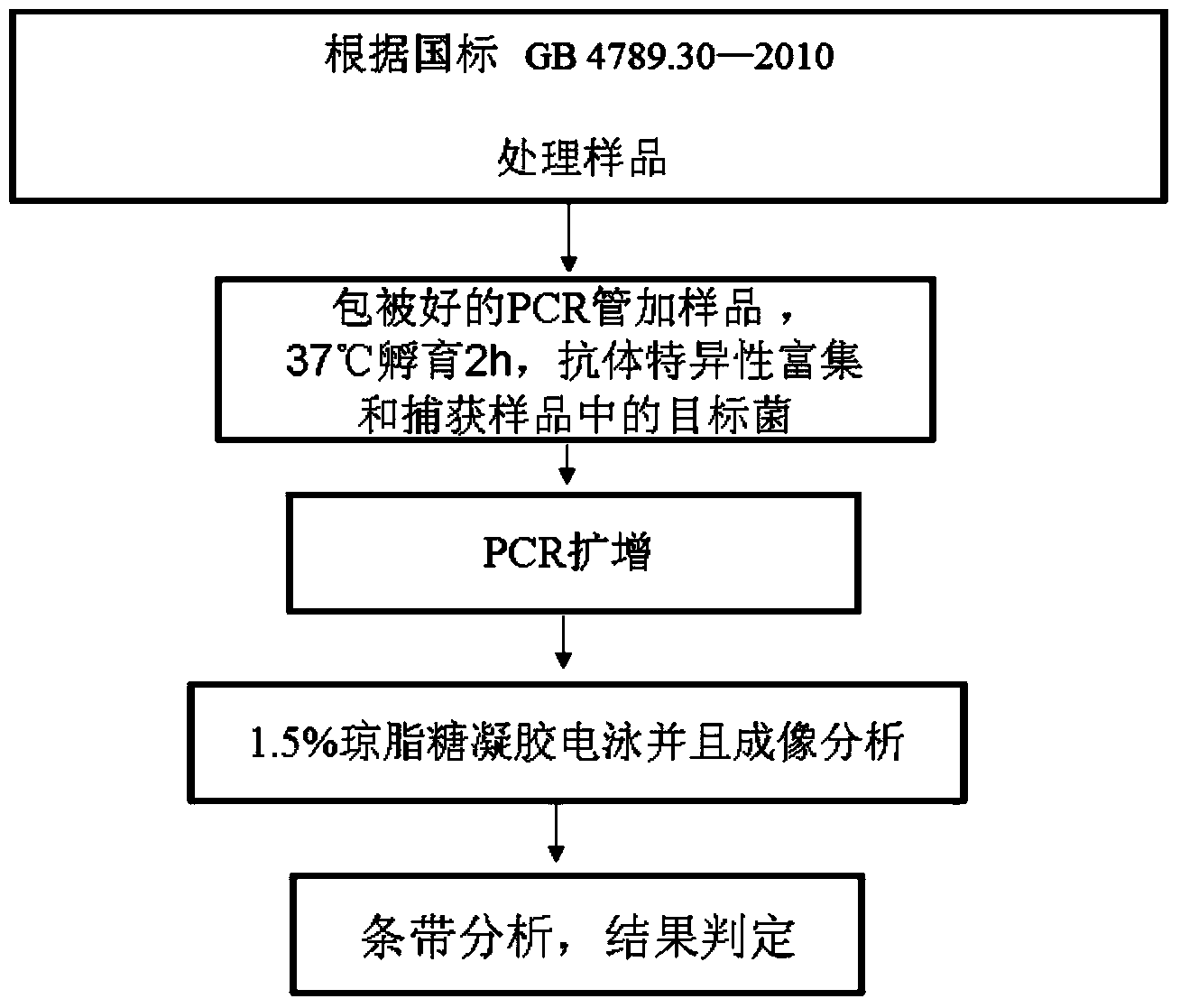
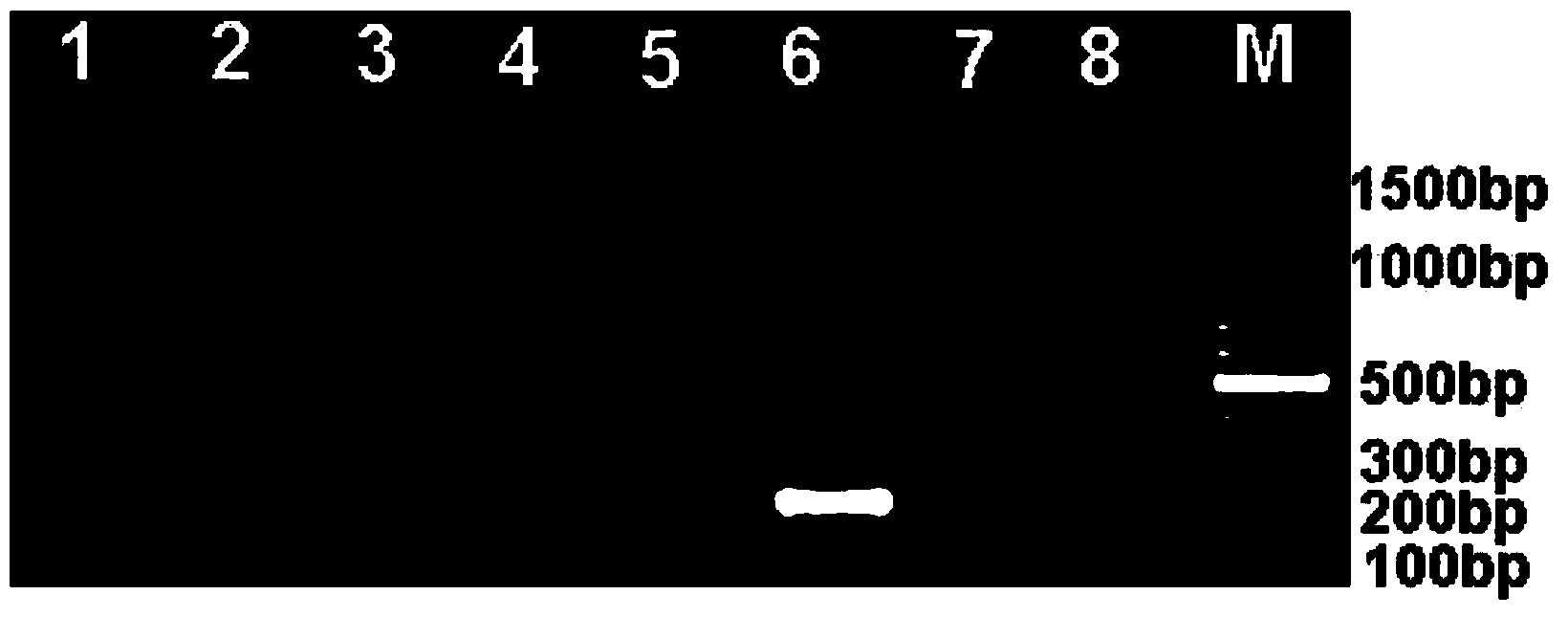
The invention discloses a specific antibody coated PCR (polymerase chain reaction) tube and an immune PCR detection kit which are used for detecting Listeria monocytogenes. The PCR reaction tube is precoated with monoclonal antibodies which can specifically enrich Listeria monocytogenes in samples. The detection kit can quickly, accurately and sensitively detect the Listeria monocytogenes from the samples like food, and the sensitivity can be 10<3>-10<4>cfu / ml and is increased by 10-100 times compared with the sensitivity of the conventional PCR. The PCR reaction tube and the immune PCR detection kit have the advantages that immunology and a molecular biological method are organically combined into a whole, enrichment and detection of Listeria monocytogenes in the samples can be realized in one PCR tube, operation is easy, cost is low, detection is quick and results are accurate.
Owner:UNIV OF SHANGHAI FOR SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com