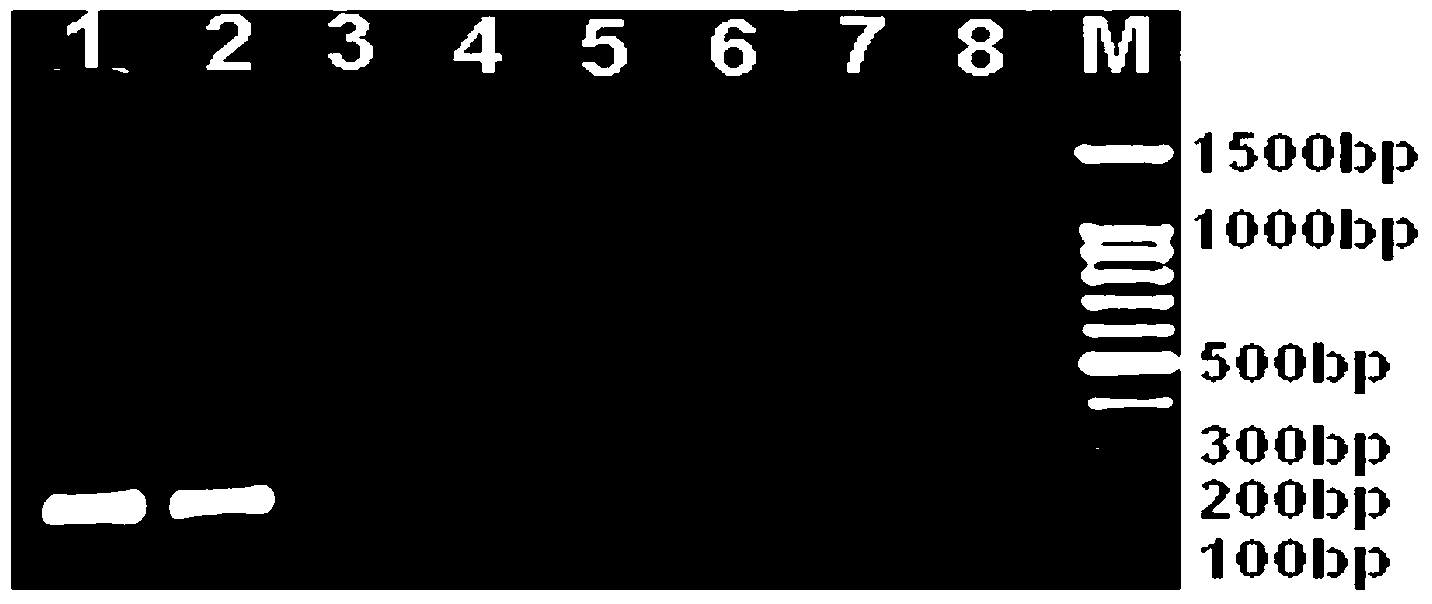Immune PCR (polymerase chain reaction) detection kit for Listeria monocytogenes
A technology for mononucleosis and Listeria, applied in the fields of biotechnology and immunology, can solve the problems of detection products relying on imports, inability to adapt to sample screening, long detection cycle, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
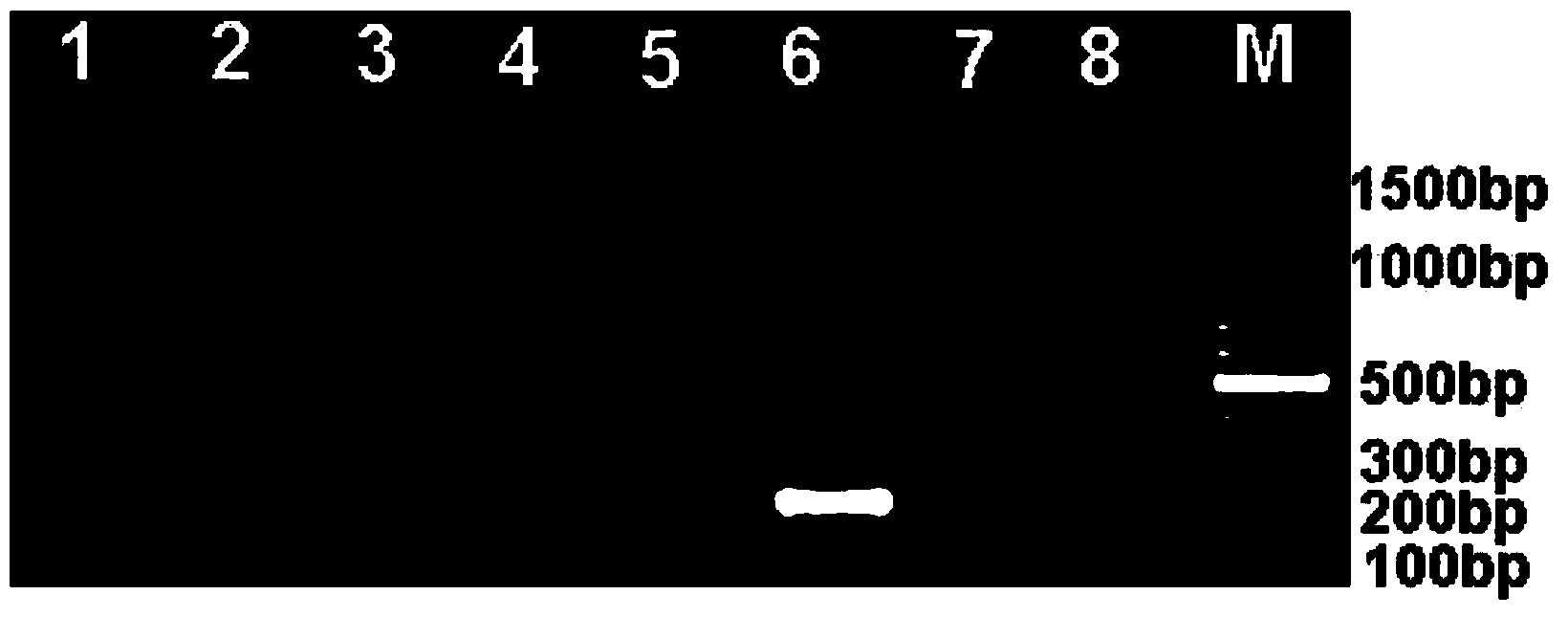
[0072] Example 1. Preparation of anti-Listeria monocytogenes monoclonal antibodies 6D4H7C8B6 and 1B4E7A6C9
[0073] 1. Preparation of immunogen and positive standard
[0074] Listeria monocytogenes (ATCC No.43251) was inoculated in Listeria broth at 37°C and shaken at 150r / min for 17h, counted, and inactivated by adding 0.3% formaldehyde solution at room temperature for 1 day. Adjust the concentration of Listeria monocytogenes (ATCC No.43251) to 5×10 with normal saline 9 CFU / ml was used as immunogen; the concentration was adjusted to 10 with normal saline 8 cfu / ml Listeria monocytogenes liquid as a positive control standard, Listeria broth as a negative control standard.
[0075] 2. Preparation of monoclonal antibodies
[0076] 1) Experimental animals: Three 8-week-old, weighing about 20 g female Balb / c mice were selected as experimental animals.
[0077] 2) Immunization method: each mouse was intraperitoneally injected with 0.2ml of immunogen, and the same dose was boosted ...
Embodiment 2
[0097] Example 2. Characterization of monoclonal antibodies 6D4H7C8B6 and 1B4E7A6C9
[0098] 1. Monoclonal Antibody Subclass Identification
[0099] 1. Antigen coating: Coat goat anti-mouse secondary antibody IgG+A+M with 0.01M PBS, 50 μl per well, coat overnight at 4°C, discard the liquid in the well the next day, and wash the plate 3 times.
[0100] 2. Blocking: add 200 μl of 1% BSA to each well, and block overnight at 4°C. Pat the board dry the next day without washing it.
[0101] 3. Add monoclonal antibody hybridoma cell supernatant, 8 microwells for each sample, 50 μl per well. Incubate for 1 hour at 37°C.
[0102] 4. After washing the plate 4 times, add specific binding rabbit anti-mouse IgG1, IgG2a, IgG2b, IgG3, IgA, IgM, κ, λ, and incubate at 37°C for 1 hour.
[0103] 5. After washing the plate 4 times, add diluted horseradish peroxidase-labeled anti-rabbit secondary antibody IgG (H+L) to each well, and incubate at 37°C for 30 minutes.
[0104] 6. After washing t...
Embodiment 3
[0117] The preparation of embodiment 3PCR reaction tube
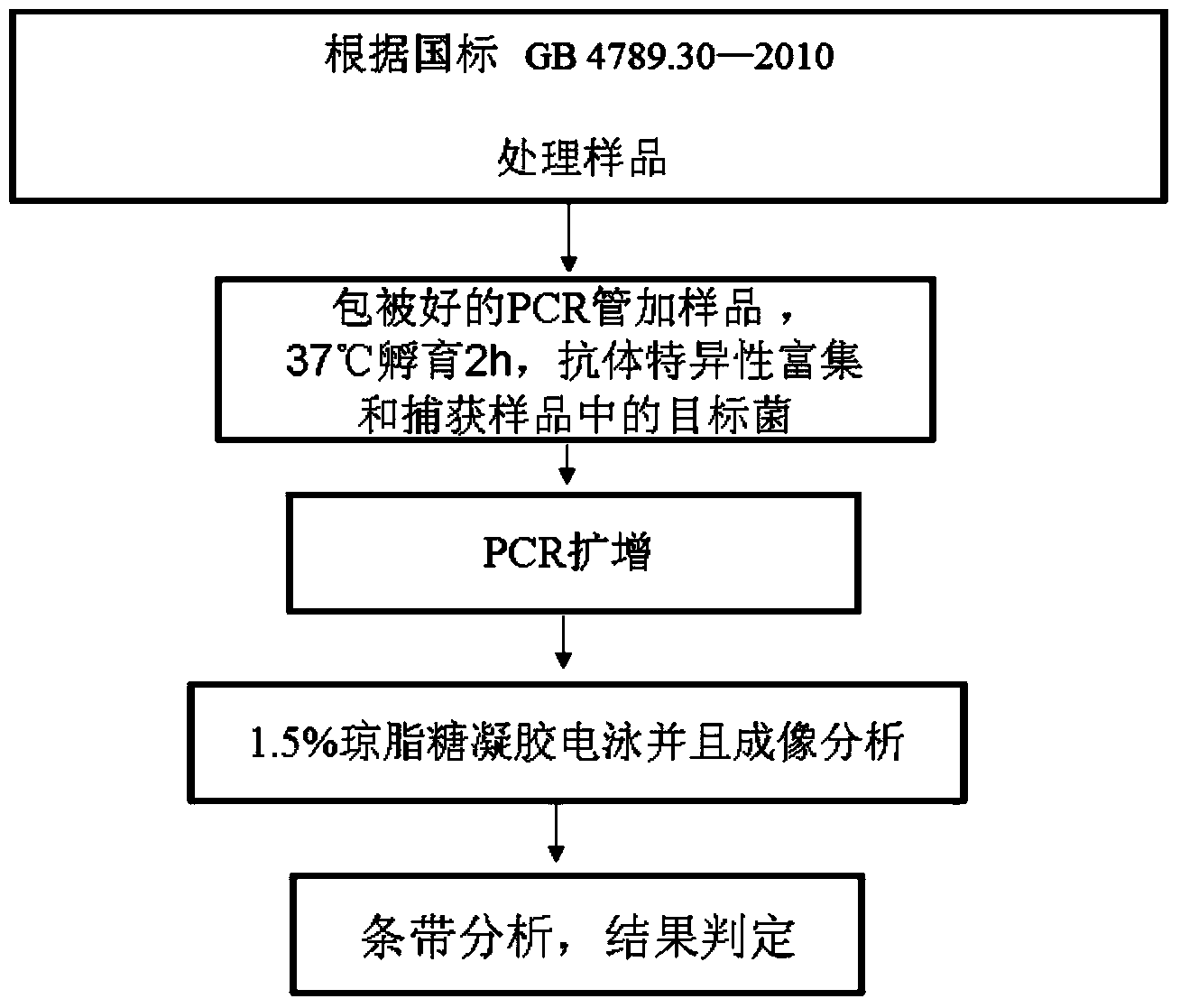
[0118] The specific coating method of the PCR reaction tube for detecting Listeria monocytogenes of the present invention is as follows:
[0119] 1. Dilute the anti-Listeria monocytogenes monoclonal antibody 6D4H7C8B6 or 1B4E7A6C9 of the present invention to 1:300 times with coating buffer, and the antibody concentration is 10 μg / mL, and add 50 uL of the diluted monoclonal antibody to each tube Immuno-PCR tubes were coated overnight at 4°C;
[0120] 2. The formula of Carbonate Coating buffer (1×) is: anhydrous sodium carbonate Na 2 CO 3 1.59g, sodium bicarbonate NaHCO 3 2.93g, sodium azide NaN 3 0.2g, dissolve in 1000ml of distilled water, adjust the pH to 9.6, and store at 4°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com