Listeria monocytogenes enzyme-linked immunosorbent assay kit
A mononuclear cell hyperplasia and enzyme-linked immunological reagent technology, applied in the fields of biotechnology and immunology, can solve the problems of inability to adapt to a large number of sample screening, complicated operations, and dependence on imports of detection products.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Example 1. Preparation of anti-Listeria monocytogenes monoclonal antibodies 1B4E7A6C9 and 6D4H7C8B6
[0057] 1. Preparation of immunogen and positive standard
[0058] Listeria monocytogenes (ATCC No.43251) was inoculated in Listeria broth, cultured at 37°C and shaken at 150r / min for 17 hours, counted, and inactivated by adding 0.3% formaldehyde solution at room temperature for 1 day. Adjust the concentration of Listeria monocytogenes (ATCC No.43251) to 5×10 with normal saline 9 CFU / ml was used as immunogen; the concentration was adjusted to 10 with normal saline 8 cfu / ml was used as a positive control standard, and Listeria broth was used as a negative control standard.
[0059] 2. Preparation of monoclonal antibodies
[0060] 1) Experimental animals: Three 8-week-old, weighing about 20 g female Balb / c mice were selected as experimental animals.
[0061] 2) Immunization method: each mouse was intraperitoneally injected with 0.2ml of immunogen, and the same dose was...
Embodiment 2
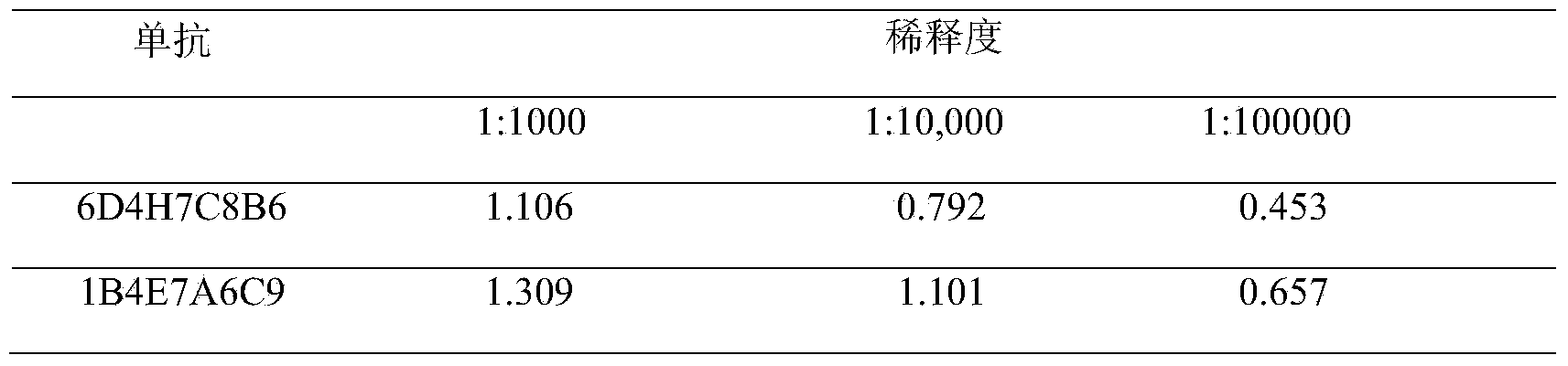
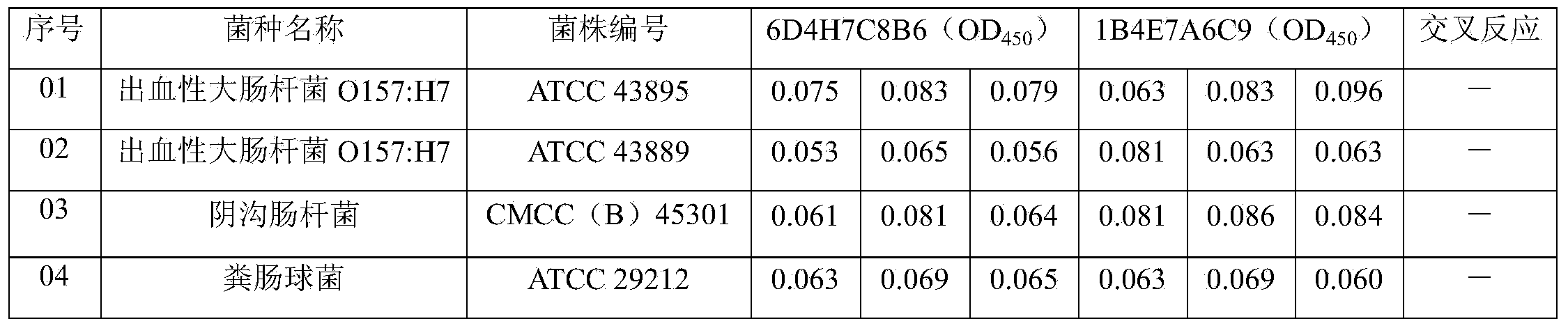
[0083] Example 2. Characterization of monoclonal antibodies 1B4E7A6C9 and 6D4H7C8B6
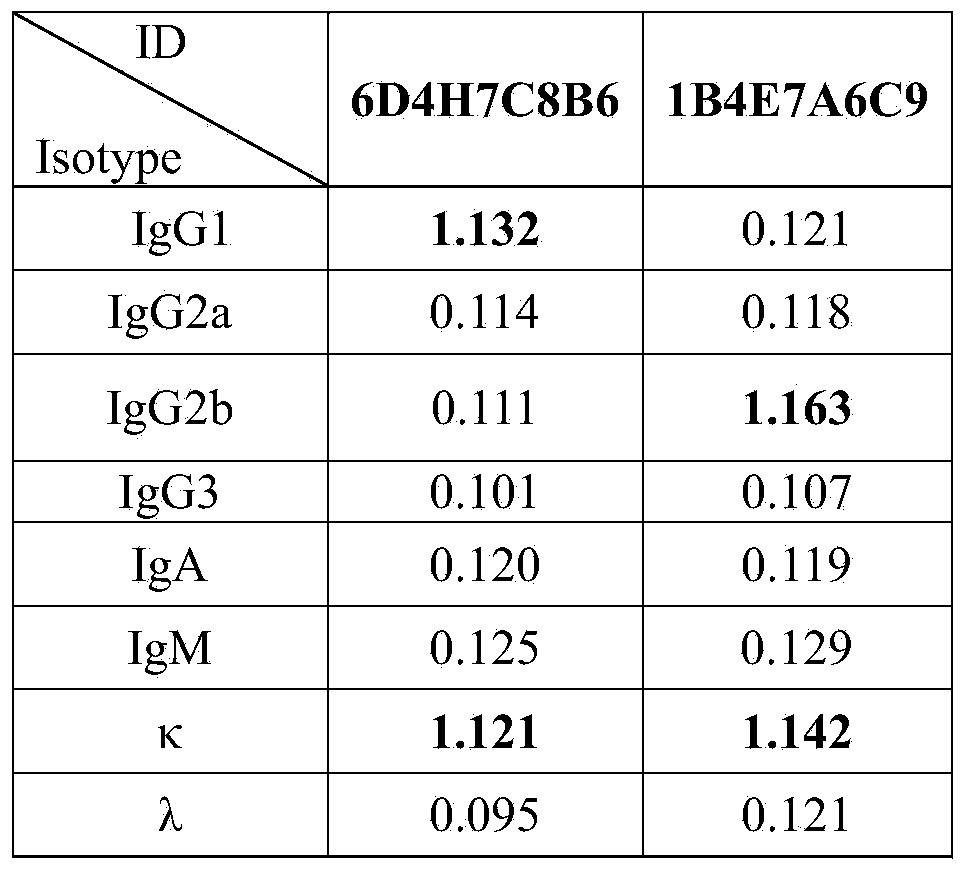
[0084] 1. Monoclonal Antibody Subclass Identification
[0085] 1. Antigen coating: Coat goat anti-mouse secondary antibody IgG+A+M with 0.01M PBS, 50 μl per well, coat overnight at 4°C, discard the liquid in the well the next day, and wash the plate 3 times.
[0086] 2. Blocking: add 200 μl of 1% BSA to each well, and block overnight at 4°C. Pat the board dry the next day without washing it.
[0087] 3. Add monoclonal antibody hybridoma cell supernatant, 8 microwells for each sample, 50 μl per well. Incubate for 1 hour at 37°C.
[0088] 4. After washing the plate 4 times, add specific binding rabbit anti-mouse IgG1, IgG2a, IgG2b, IgG3, IgA, IgM, κ, λ, and incubate at 37°C for 1 hour.
[0089] 5. After washing the plate 4 times, add diluted horseradish peroxidase-labeled anti-rabbit secondary antibody IgG (H+L) to each well, and incubate at 37°C for 30 minutes.
[0090] 6. After washing t...
Embodiment 3
[0104] Example 3. The composition, preparation and application of the ELISA kit for detecting Listeria
[0105] 1. The enzyme-linked immunosorbent assay kit consists of the following materials:
[0106] (1) ELISA plate with pre-coated antibody: Dilute with 0.02M acetate buffer (pH 2.0) solution, coat 96-well ELISA plate with anti-Listeria monoclonal antibody 1B4E7A6C9, 100 μl per well. Incubate overnight at 4°C, block and wash according to conventional ELISA methods.
[0107] (2) Listeria positive control standard and negative control standard.
[0108] (3) Anti-Listeria monoclonal antibody 6D4H7C8B6 labeled with horseradish peroxidase.
[0109] (4) Enzyme-labeled antibody diluent: 0.01M PBS, pH7.6.
[0110] (5) 10× concentrated lotion: 0.1M phosphate buffer containing 0.5% Tween-20 and 0.2% sodium azide, pH 7.4, just dilute the concentrated lotion 10 times before use.
[0111] (6) Chromogenic solution A and chromogenic solution B. Mix equal volumes of liquid A and liquid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com