Cell electrode and electrochemical cell therewith
a cell electrode and electrode active material technology, applied in the field of cell electrodes and electrochemical cells therewith, can solve the problems of insufficient long-term stability under high temperature atmosphere, accelerated deterioration, and aggravated deterioration, so as to prevent peroxidation-perreduction deterioration of an electrode active material, improve cycle properties, and improve electrode performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
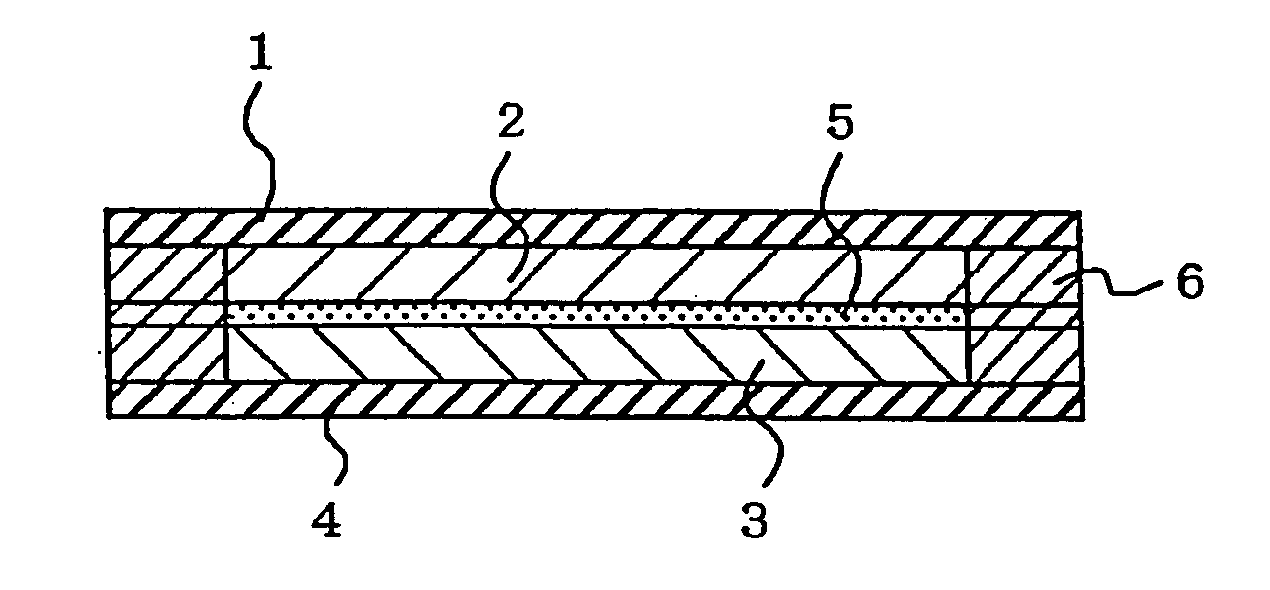
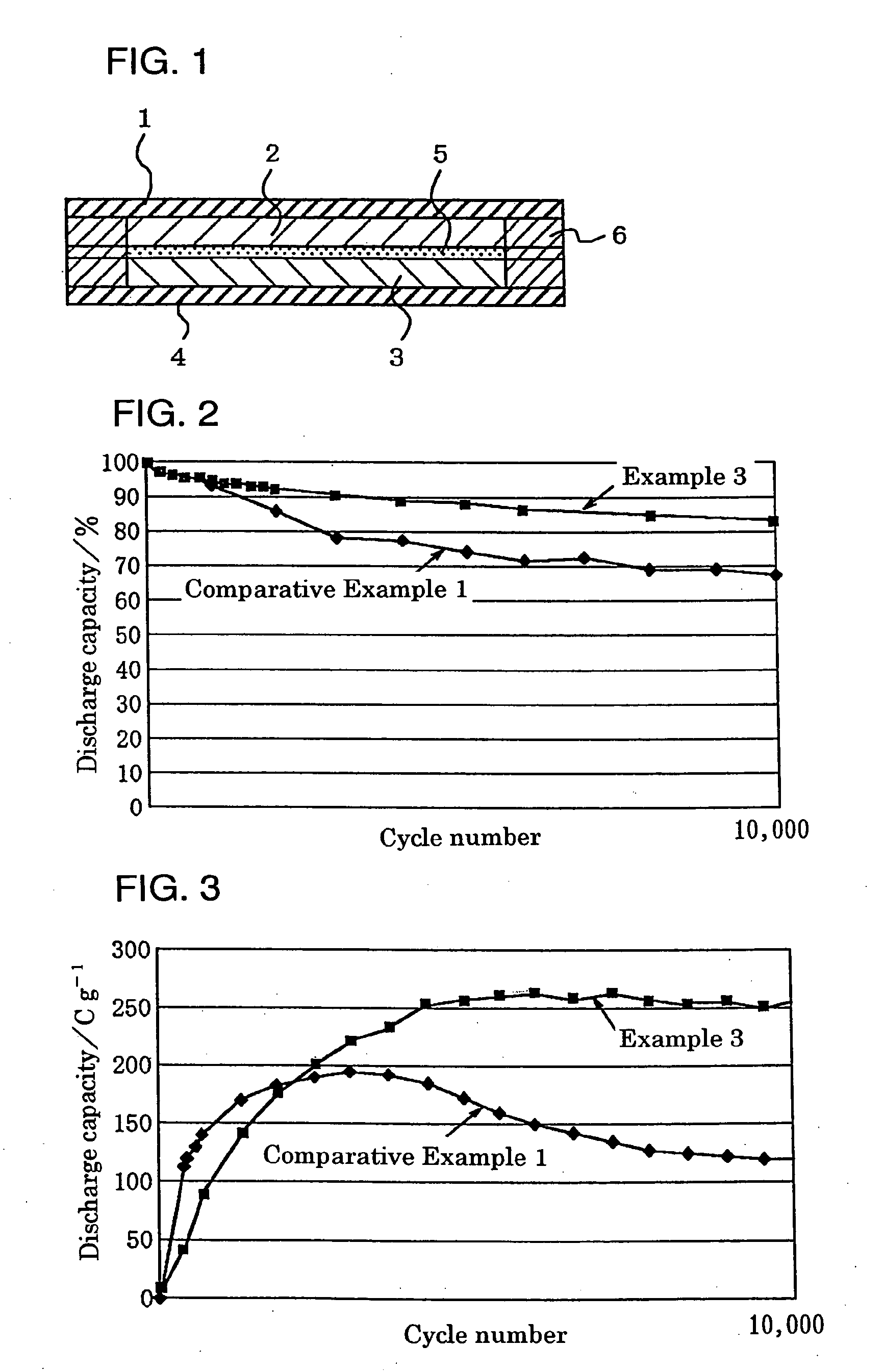
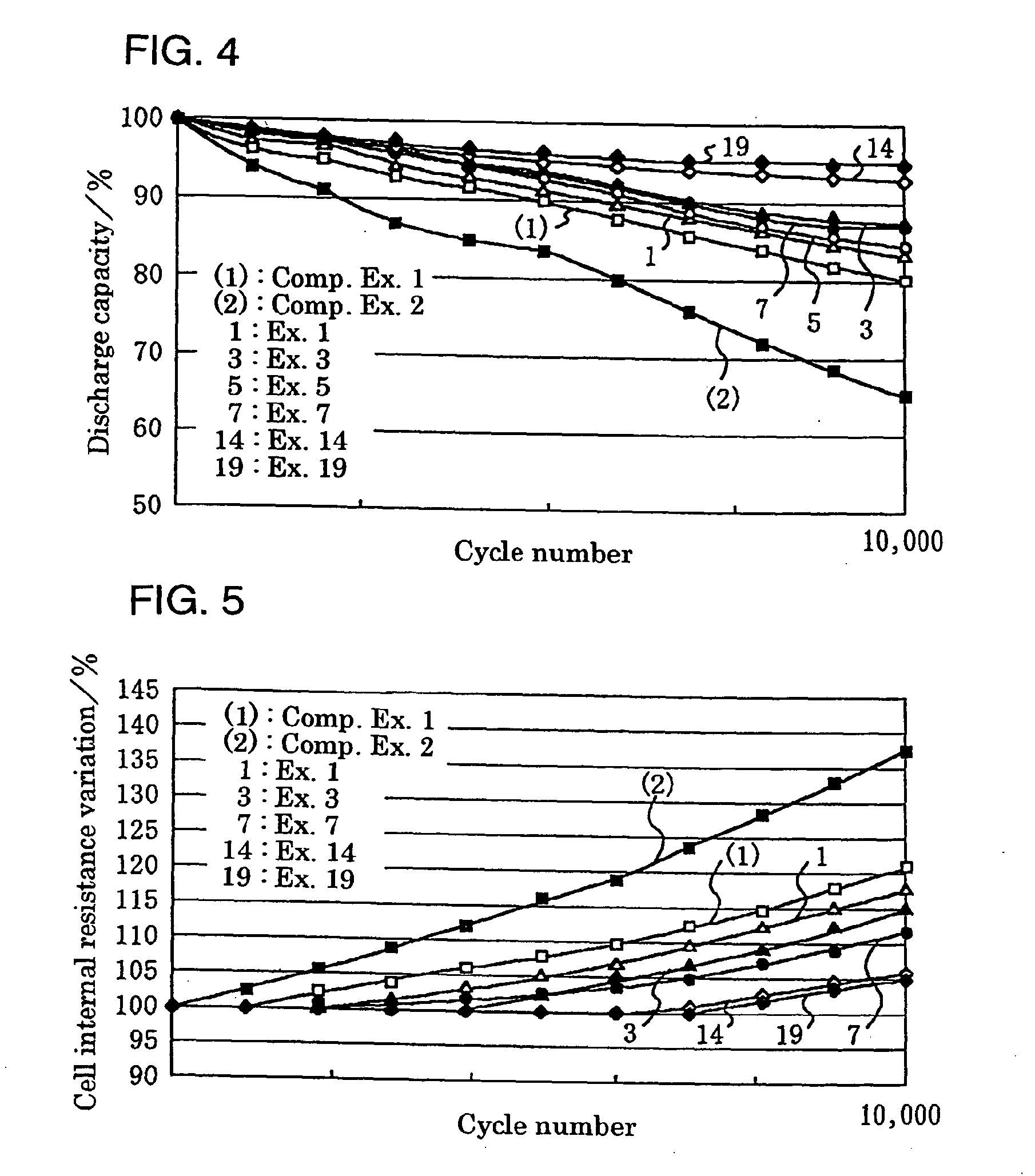
[0071] A positive electrode used was prepared as follows. To indole trimer 69 wt % as an active material were added 23 wt % of vapor growth carbon (VGCF) as a conductive auxiliary and 8 wt % of a polyfluorovinylidene (average molecular weight: 1100) as an electrode molding component. To 100 wt % of the mixture was added 5 wt % of imidazole. The resultant mixture was stirred and blended in a blender and then molded by a hot press into a solid electrode having a desired size, which was used as a positive electrode 2.
[0072] A negative electrode used was prepared as follows. To polyphenylquinoxaline 75 wt % as an active material were added 25 wt % of carbon black (K.B.600) as a conductive auxiliary. To 100 wt % of the mixture was then added 5 wt % of imidazole. The resultant mixture was stirred and blended in a blender and then molded by a hot press into a solid electrode having a desired size, which was used as a negative electrode 3.
[0073] An electrolytic solution used was a 20 wt %...
example 2
[0076] A positive electrode was prepared as described in Example 1 without adding imidazole. A negative electrode was prepared as described in Example 1 except adding 20 wt % of imidazole. A battery was formed as described in Example 1, except these electrodes were used.
example 3
[0077] A positive electrode was prepared as described in Example 1 except adding 20 wt % of imidazole. A negative electrode was prepared as described in Example 1 except adding 20 wt % of imidazole. A battery was formed as described in Example 1, except these electrodes were used.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| wt % | aaaaa | aaaaa |
| wt % | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com