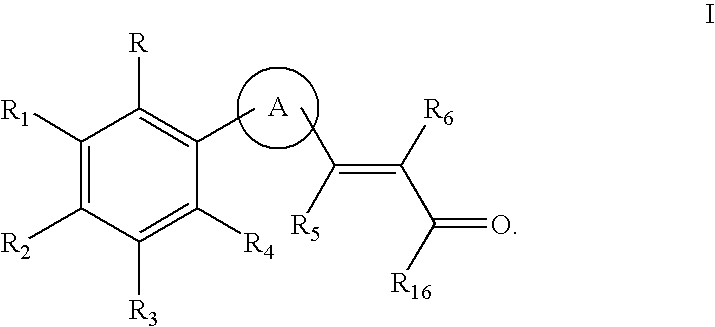
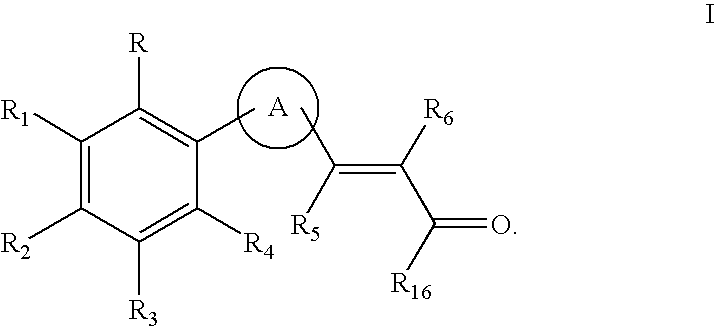
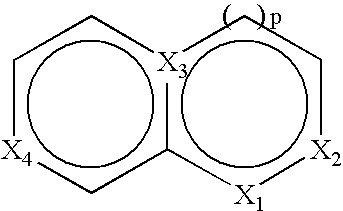Retinoid x receptor modulators
a technology of retinoids and receptors, applied in the field of retinoids with receptor modulators, can solve the problems of many undesirable side effects of retinoids, skin irritation, lipid and bone toxicity, etc., and achieve the effect of fewer side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
3-[5-(2-Hydroxy-3-tert-butyl-5-ethylphenyl)-benzo[b]furan-2-yl]-but-2-enoic acid
[0292]
A. 2-Hydroxy-5-(5-ethyl-3-tert-butyl-2-methoxymethoxyphenyl)-benzaldehyde
[0293]
[0294] To a mixture of 91 mg (0.078 mmol, 5%) of Pd(PPh3)4 and 252 mg (1.26 mmol) of 5-bromo salicylaldehyde in 10 mL of dry toluene was added 685 mmol (2.5 mmol, 2 equivalents) of 2-methoxymethoxy-3-tert-butyl-5-ethyl phenylboronic acid diluted in 5 mL of ethanol followed by 1.3 mL of a 2N aqueous solution of Na2CO3. The mixture was stirred at reflux for 3 hours and after cooling extracted with ethyl acetate. The organic layer was dried over MgSO4 and evaporated under reduced pressure. The residual oil was purified over silica gel (eluent: ethyl acetate / hexane:10 / 90) to give 333 mg (0.969 mmol, yield: 77%) of 2-hydroxy-5-(5-ethyl-3-tert-butyl-2-methoxymethoxyphenyl)-benzaldehyde a pale brown oil. 1H NMR (CDCl3), δ: 11.04 (s, 1H), 9.95 (s, 1H), 7.75 (dd, J=8.9, 2.1 Hz, 1H), 7.74 (d, J=2.1 Hz, 1H), 7.19 (d, J=2.1 Hz, 1H...
example 2
2-Fluoro-3-[5-(2-methoxy-3,5-diisopropylphenyl)-benzo[b]furan-2-yl]-but-2-enoic acid
[0302]
A. 2-Acetyl-5-bromo benzo[b]furan
[0303]
[0304] A mixture of 5.0 g (24.9 mmol) of 5-bromo-salicylaldehyde, 3.0 g (32.3 mmol, 2.6 mL) of chloroacetone and 12 g (37.2 mmol) of Cs2CO3 in 30 mL of dry DMF was heated to 60° C. overnight. After cooling at room temperature, water (100 mL) was added and the mixture was extracted with ethyl acetate. The organic layer was dried over MgSO4 and the solvents were removed under reduced pressure. The crude product was directly recrystallized from hexane to afford 4.14 g (17.3 mmol, yield: 70%) of 2-acetyl-5-bromo benzo[b]furan as a pale orange crystal. 1H NMR (CDCl3), δ: 7.84 (d, J=1.9 Hz, 1H), 7.56 (dd, J=9.0, 2.1 Hz, 1H), 7.45 (d, J=8.9 Hz, 1H), 7.42 (s, 1H), 2.60 (s, 3H).
B. 2-Acetyl-5-[(2-methoxy-3,5-diisopropyl)-phenyl]benzo[b]furan
[0305]
[0306] To a mixture of 57 mg (0.049 mmol, 5%) of Pd(PPh3)4, 362 mg (1.26 mmol) of 2-acetyl-5-bromobenzo[b]furan and 2...
example 3
2-Fluoro-3-[7-(2-propoxy-3-tert-butyl-5-ethylphenyl)-benzo[b]furan-2-yl]-but-2-enoic acid ethyl ester
[0310]
A. 2-Acetyl-7-bromo benzo[b]furan
[0311]
[0312] A mixture of 5.0 g (24.9 mmol) of 3-bromo-salicylaldehyde, 3.0 g (32.3 mmol, 2.6 mL) of chloroacetone and 12 g (37.2 mmol) of Cs2CO3 in 30 mL of dry DMF was heated to 60° C. overnight. After cooling at room temperature, water (100 mL) was added and the mixture was extracted with ethyl acetate. The organic layer was dried over MgSO4 and the solvents were removed under reduced pressure. The crude product was directly recrystallized from hexane to afford 2-acetyl-7-bromo benzo[b]furan as a pale orange crystal.
B. 2-Acetyl-7-(2-propoxy-3-tert-butyl-5-ethylphenyl)benzo[b]furan
[0313]
[0314] To a mixture of 57 mg (0.049 mmol, 5%) of Pd(PPh3)4, 362 mg (1.26 mmol) of 2-acetyl-7-bromobenzo[b]furan and 2.52 mmol of 2-propoxy-3-tert-butyl-5-ethyl phenylboronic acid in 10 mL of toluene, and 5 mL of ethanol was added 1.3 mL of a 2N Na2CO3 aqueo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| weight percent | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com