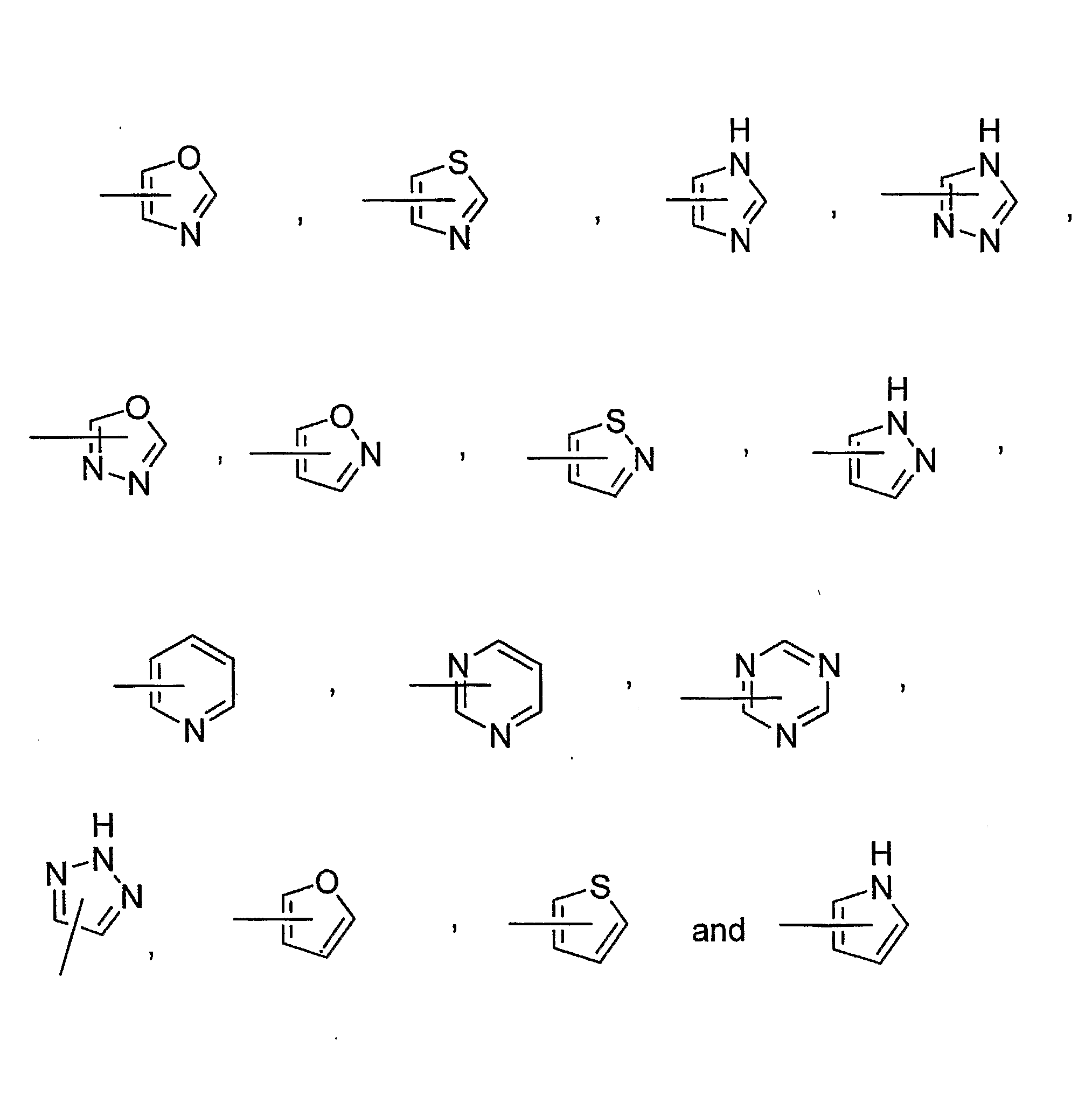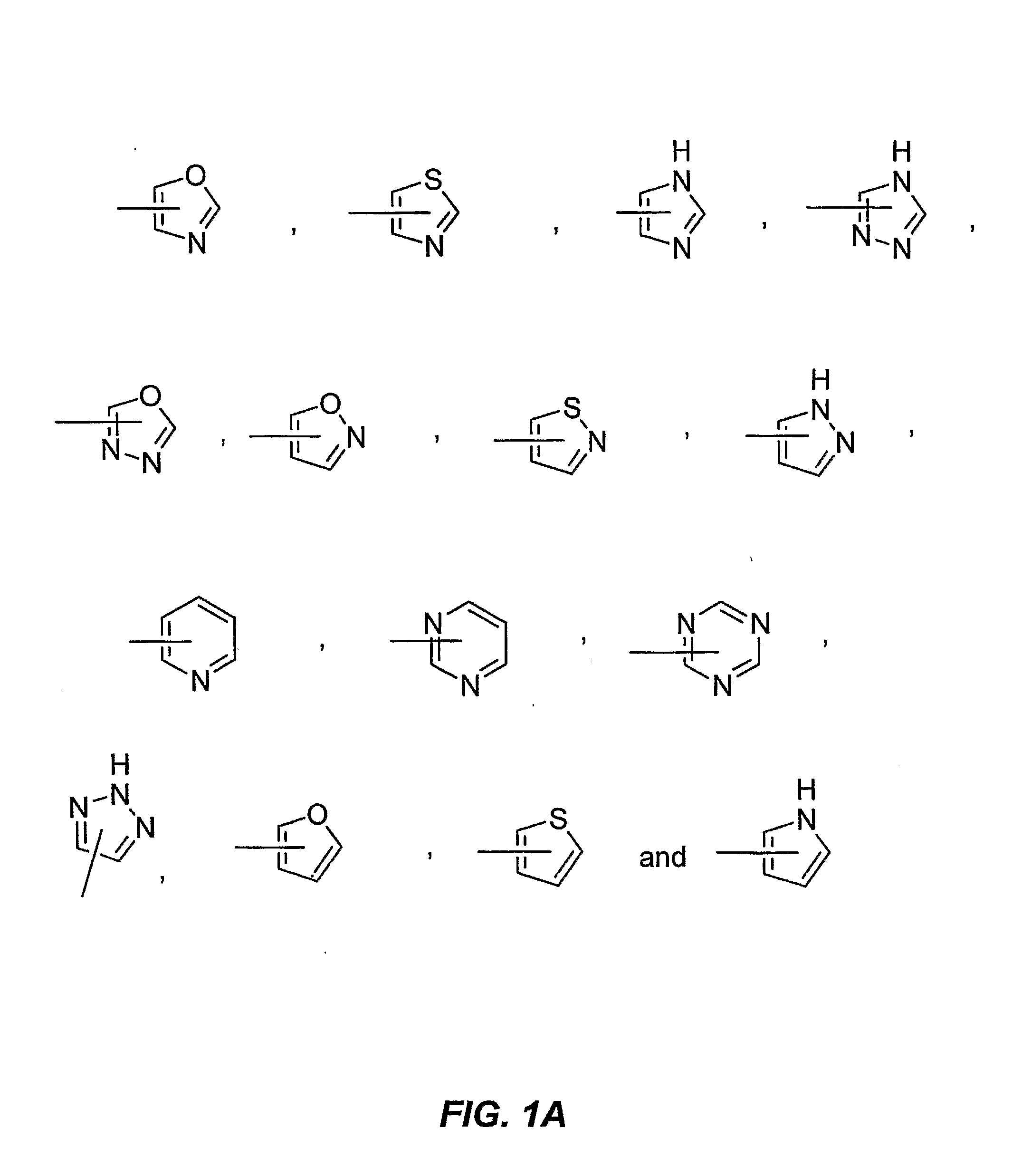Methods For Avoiding Edema in the Treatment of Metabolic, Inflammatory, and Cardiovascular Disorders
a metabolic, inflammatory, cardiovascular disorder technology, applied in the direction of immunological disorders, metabolism disorders, extracellular fluid disorders, etc., can solve the problems of increased risk of cerebrovascular and cardiovascular events, increased risk of edema, etc., to reduce cardiac afterload, reduce cardiac afterload, and improve hemodynamic
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiment 1
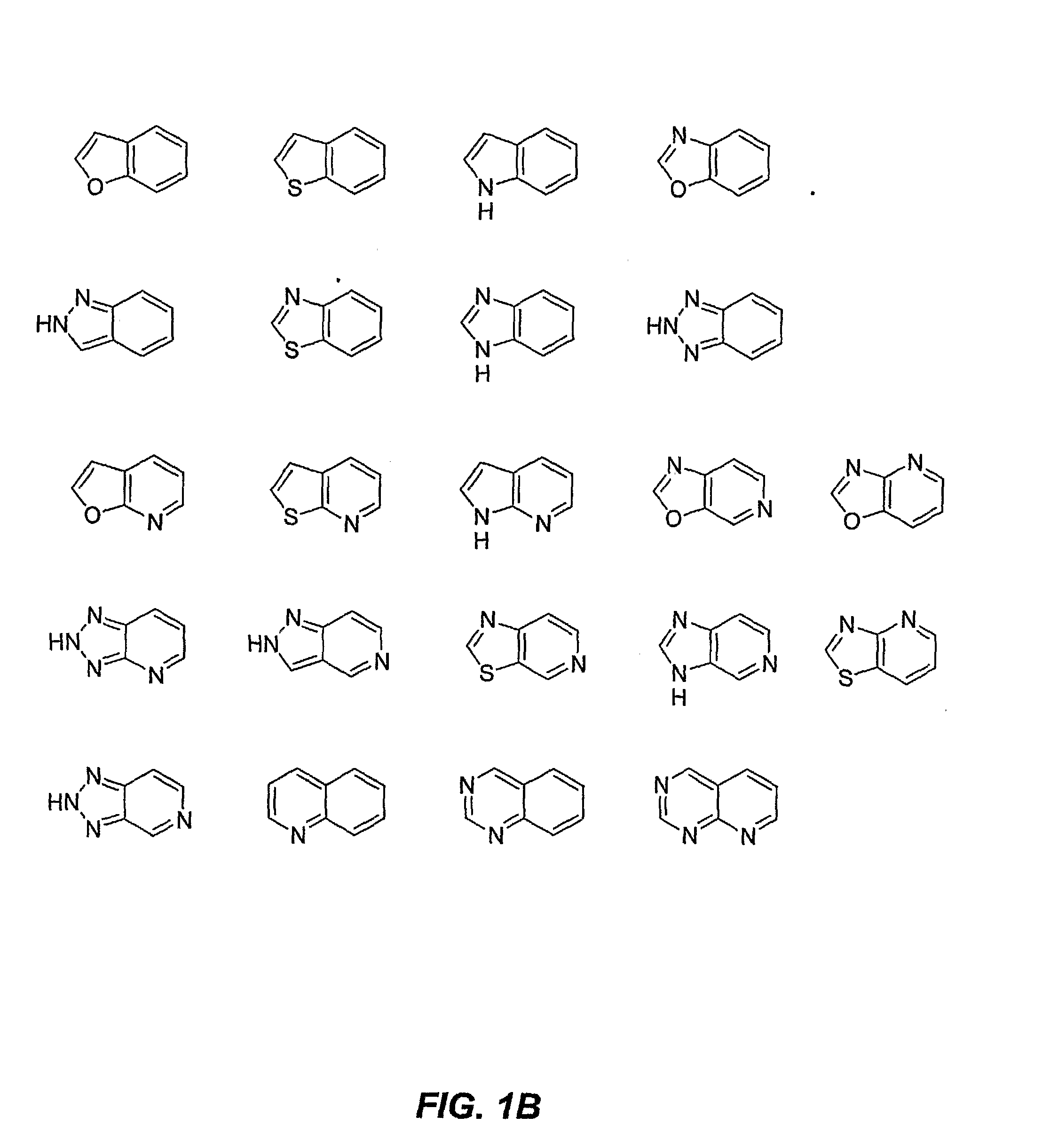
[0146]A compound having a formula selected from the group consisting of:
wherein[0147]X is a member selected from the group consisting of O, S, SO, SO2 and NR, wherein R is H, (C1-C8)alkyl, CORa, COORa and CONRaRb wherein Ra and Rb are each independently selected from the group consisting of H and (C1-C8)alkyl;[0148]Y is a member selected from the group consisting of CH2ORc, CO2Rc, CHO, CONRcRm, CH(═NRc), CH(═NORc) and carboxylic acid surrogates, wherein Rc is a member selected from the group consisting of H, (C1-C8)alkyl, (C3-C8)alkenyl, (C3-C8)alkynyl, (C3-C7)cycloalkyl, (C4-C8)cycloalkyl-alkyl, aryl, aryl(C1-C8)alkyl and (C1-C8)alkylene-Z, wherein Z is selected from the group consisting of CORd, COORd, NRdRe, NRdCONReRf, NRdCORe, NRdCOORe and CONRdRe wherein Rd, Re and Rf are each independently selected from the group consisting of H, (C1-C8)alkyl and phenyl, or optionally two of Rd, Re and Rf when attached to the same nitrogen atom are combined to form a five- or six-membered rin...
embodiment 2
[0155]A compound of embodiment 1, wherein Y is selected from the group consisting of CH2ORc, CO2Rc, tetrazol-5-yl, CONHSO2Rn and CHO.
embodiment 3
[0156]A compound of embodiment 1, wherein Y is selected from the group consisting of CH2ORc, tetrazol-5-yl, CONHSO2Rn and CO2Rc
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com