Epitopes Related to Coeliac Disease
a coeliac disease and epitope technology, applied in the field of epitopes related to coeliac disease, can solve the problems of toxic gluten proteins in wheat, rye, barley and in some cases oats, and achieve the effect of high throughpu
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0210]The invention is illustrated by the following nonlimiting Examples:
Initial Gliadin Epitope Screening Library
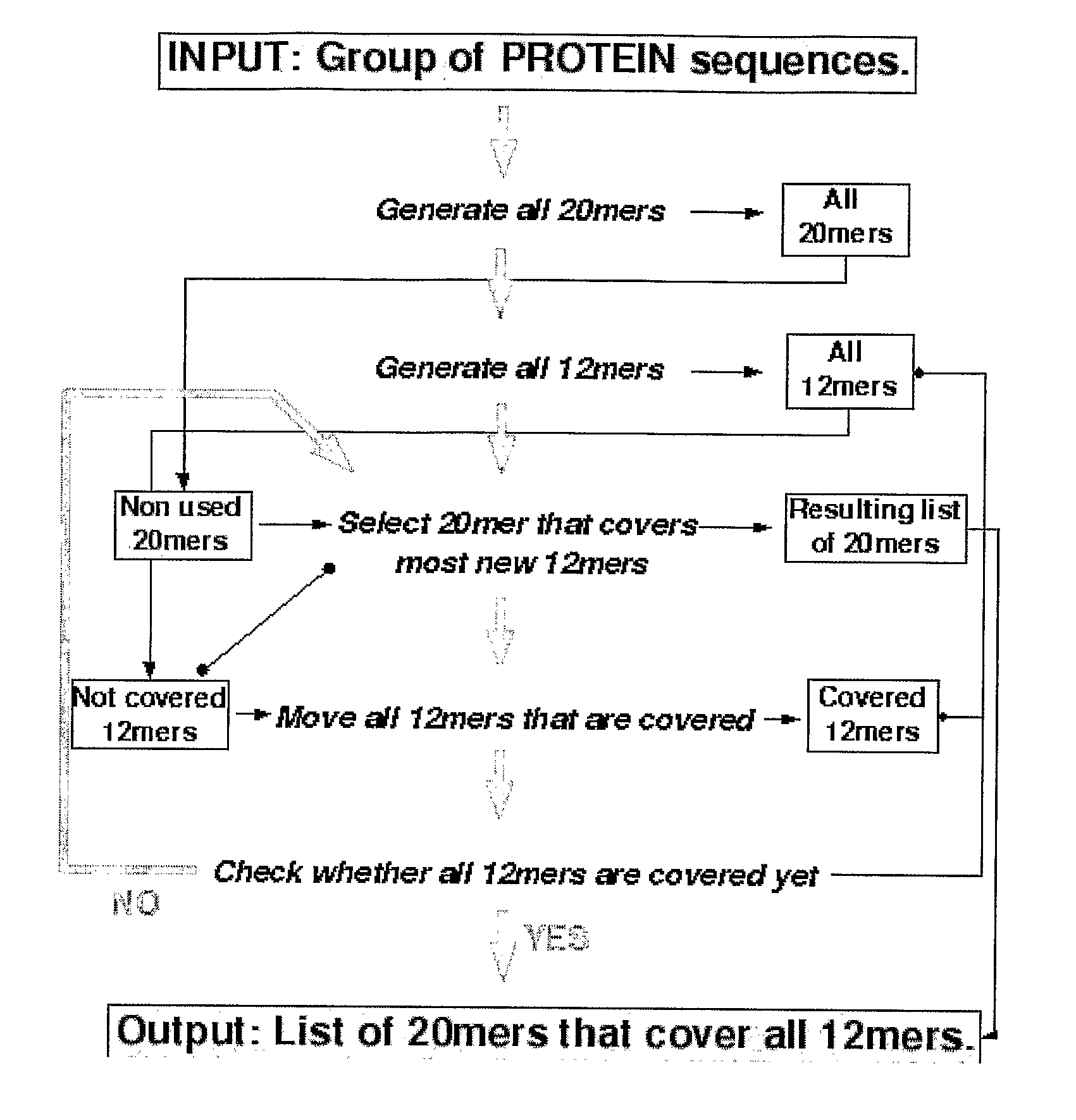
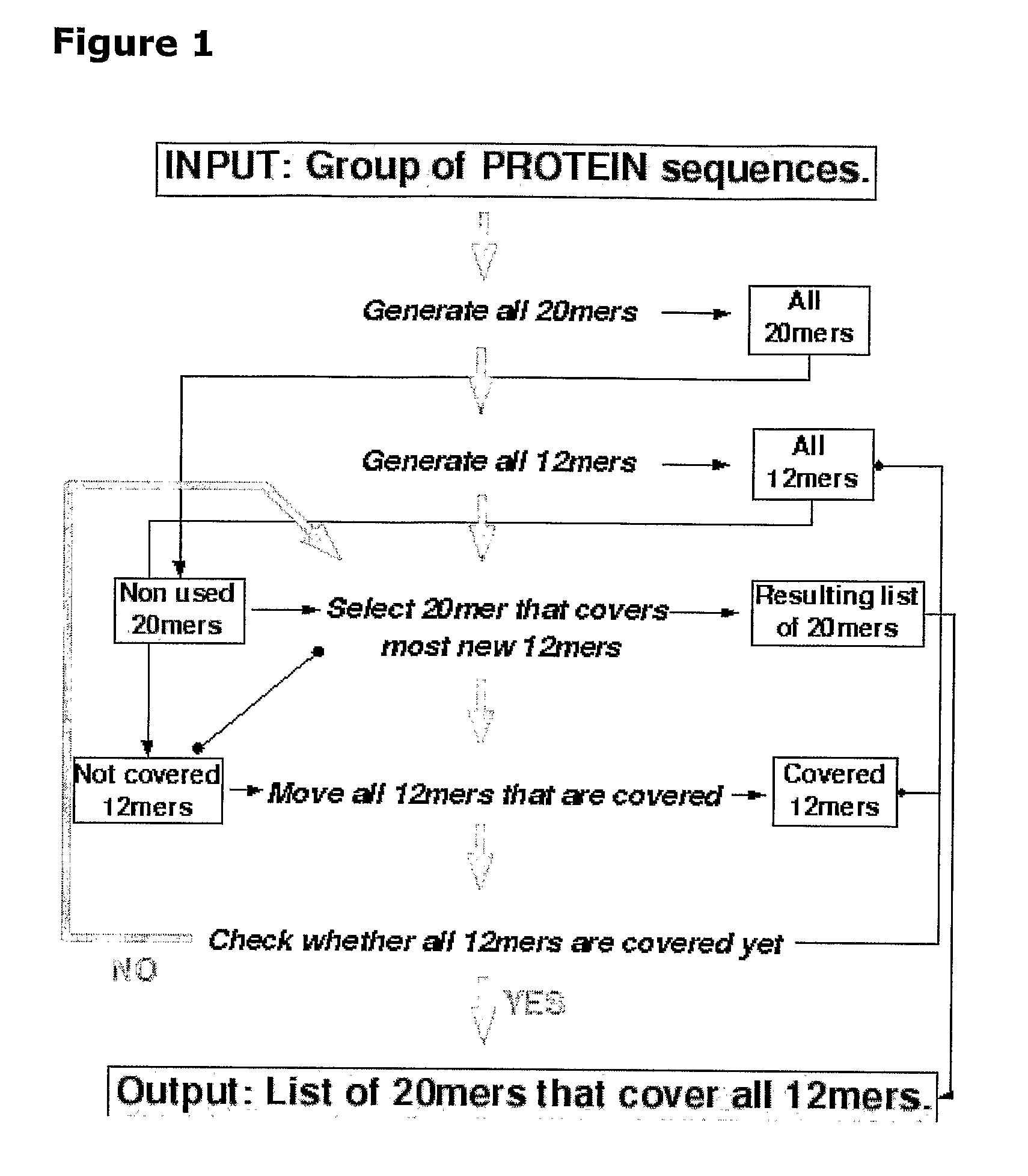
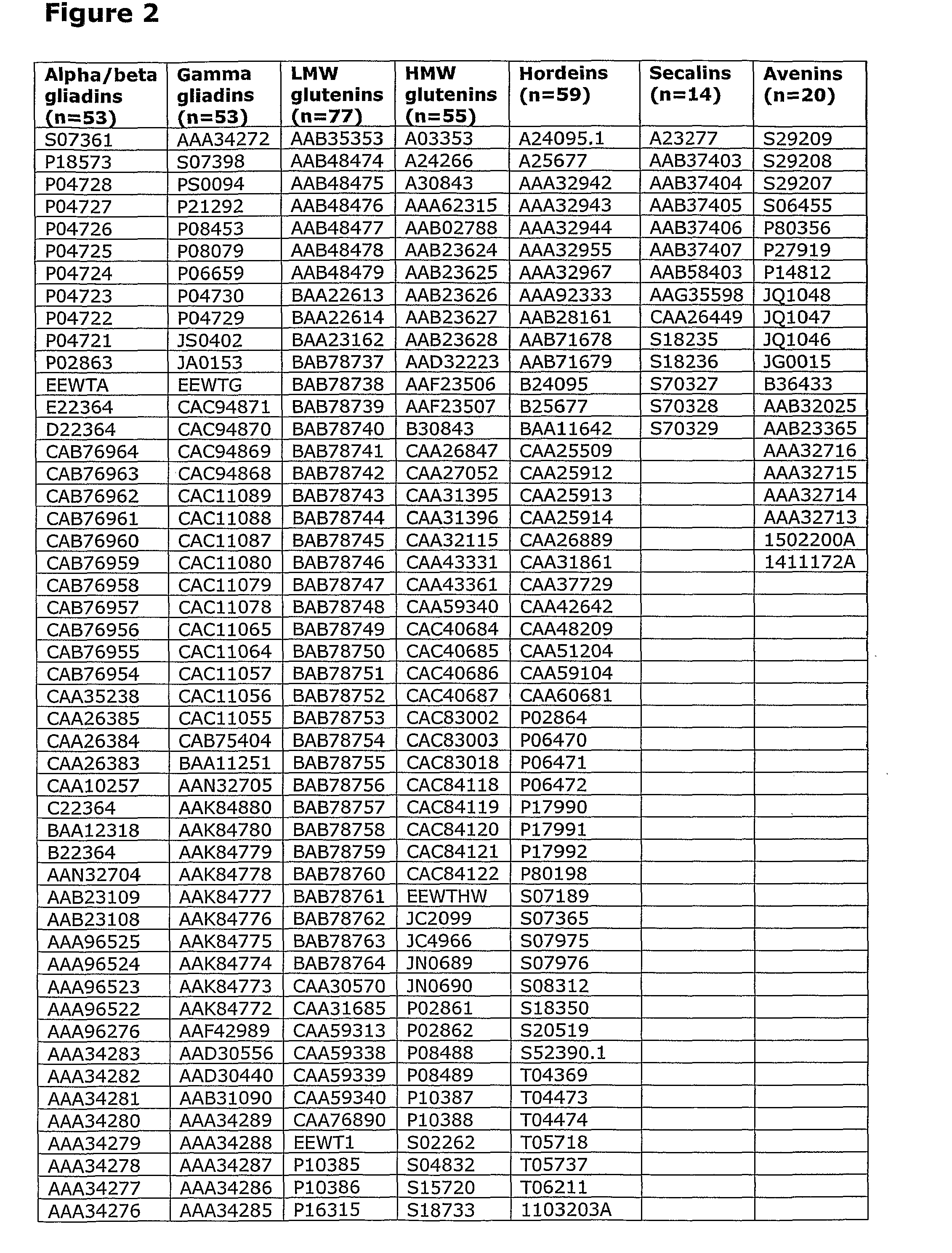
[0211]In initial experiments involving 29 HLA-DQ2+ individuals with coeliac disease on long-term gluten free diet, interferon-gamma ELISPOT assays were used to screen a previous Pepset (described in WO 03 / 104273, which is incorporated herein by reference) initially as pools of peptides and then in 15 subjects as individual peptides with and without deamidation by tTG. This Pepset library consisted of 652 20mer gliadin peptides spanning all unique 12mers contained within all Genbank entries described as wheat gliadins found in September 2001. This Pepset library was designed “manually” from gene-derived protein sequences aligned using ClustalW software (MegAlign) arranged into phylogenetic groupings.
[0212]Approximately 0.6 micromole of each of 652 of the 20mers was provided. Two marker 20mer peptides were included in each set of 96 (VLQQHNIAHGSSQVLQESTY—peptide 161, and I...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| length of time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com