Process for Decellularizing Soft-Tissue Engineered Medical Implants, and Decellularized Soft-Tissue Medical Implants Produced
a technology of engineered medical implants and soft tissues, which is applied in the direction of biocide, prosthesis, drug compositions, etc., can solve the problems of proteolytic degradation of the matrix structure of the processed tissues, prohibitively time-consuming prior art processing methods, and easy requiring numerous days
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
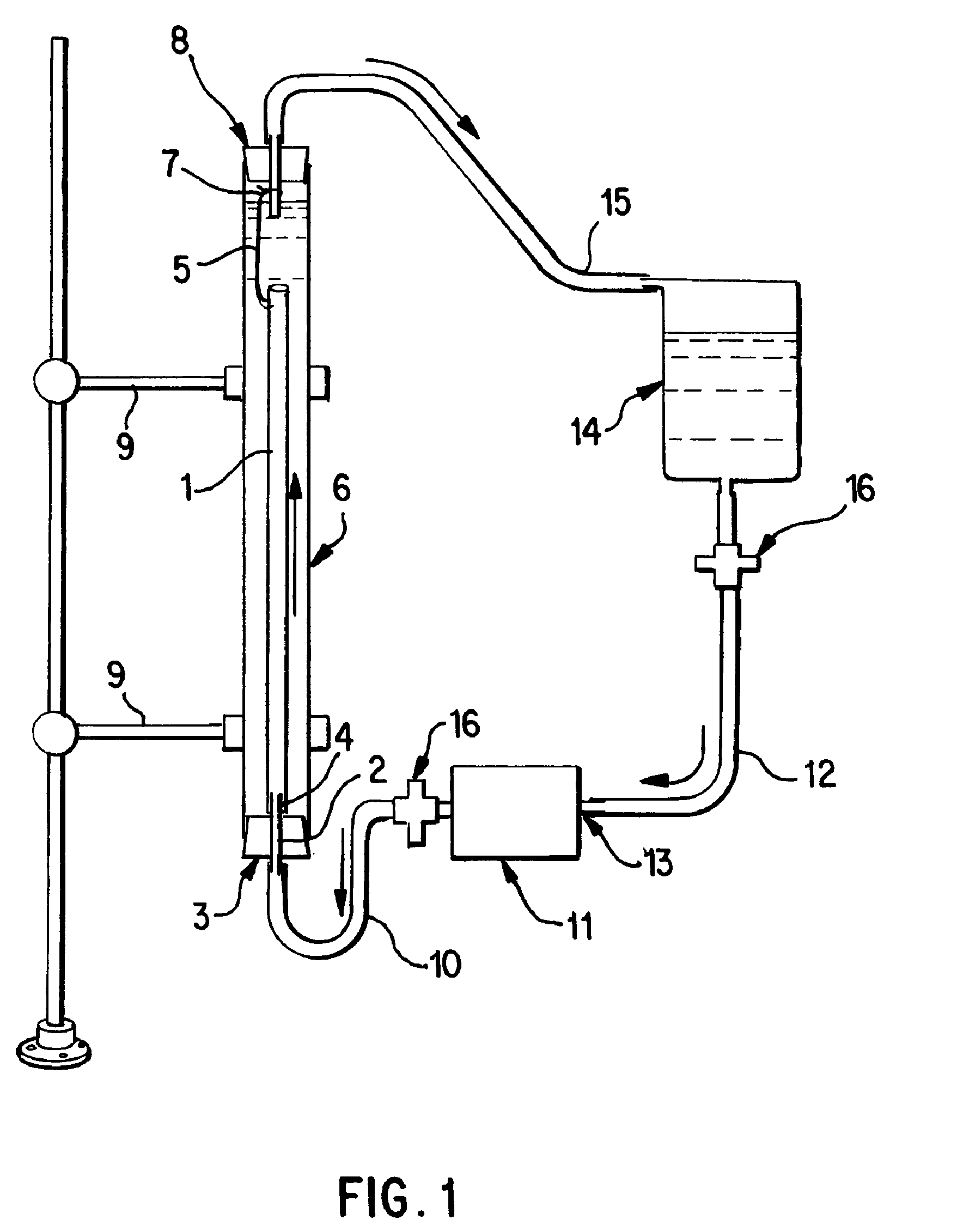
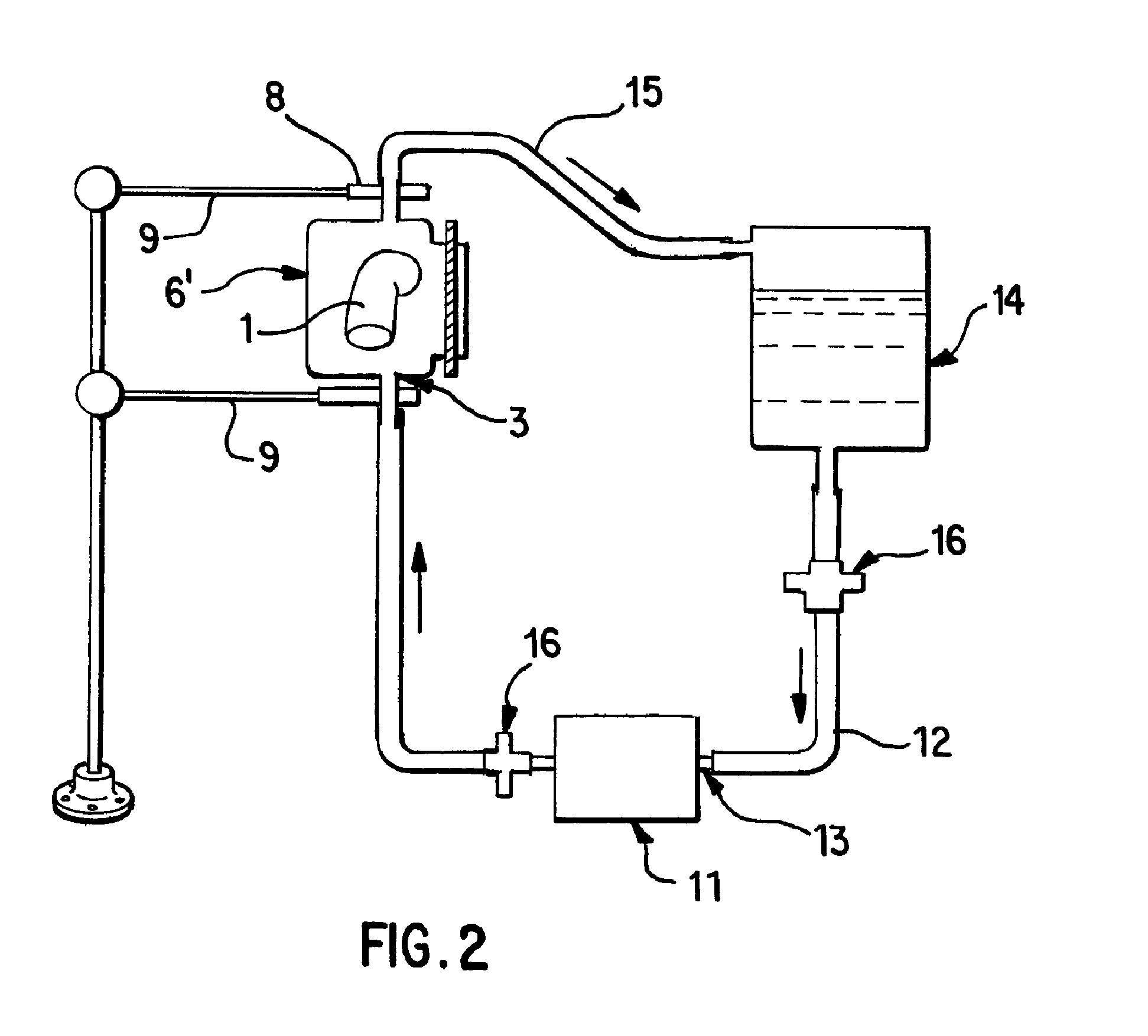
[0058]Saphenous vein tissues (two) from each leg of an acceptable human donor were carefully dissected under sterile conditions to remove all visible fat deposits and the side vessels were tied off using nonresorbable suture materials such that the ties did not occur in close proximity to the long run of the vessel. Sutures can restrict the decellularization process and the tissues under the sutures were removed following decellularization. For long vein grafts (40-60 cm) (FIG. 1), the distal ends of the veins were cannulated onto the ribbed attachment of the inlet port(s) and single sutures used to secure each vein. Additional suture lines were attached to the proximal ends of the veins. The veins were then removed from the dissecting solution (ultrapure water containing 50 mM Tris-HCl (pH 7.2), 5 μm EDTA, and one or more antibiotics) and transferred to the processing vessel which had been temporarily inverted. The second suture line along with the vein was passed through the proce...
example 2
[0059]Saphenous vein tissues (two) from each leg of an acceptable human donor were carefully dissected under sterile conditions to remove all visible fat deposits and side vessels were tied off using nonresorbable suture materials such that the ties did not occur in close proximity to the long run of the vessel. Sutures can restrict the decellularization process and the tissues under the sutures were removed following decellularization. For these long vein grafts (33 and 28 cm) (FIG. 1), the distal ends of the veins were cannulated onto the ribbed attachment of the inlet port(s) and single sutures used to secure each vein. Additional suture lines were attached to the proximal ends of the veins. At this point, the veins were removed from the dissecting solution (ultrapure water containing 50 mM Tris-HCl (pH 7.2), 5 mM EDTA, and one or more antibiotics and transferred to the processing vessel which had been temporarily inverted. The second suture line along with the vein was passed th...
example 3
[0060]Internal mammary artery tissues (two) from an acceptable human donor were carefully dissected under sterile conditions to remove all visible fat deposits and side vessels were tied off using nonresorbable suture materials such that the ties did not occur in close proximity to the long run of the vessel. Sutures can restrict the decellularization process and the tissues under the sutures were removed following decellularization. For short artery grafts (11 and 8 cm) (FIG. 1), one end of each artery were cannulated onto the ribbed attachment of the inlet port(s) and single sutures used to secure each arteries. The arteries were then removed from the dissecting solution (ultrapure water containing 50 mM Tris-HCl (pH 7.2), 5 mM EDTA, and one or more antibiotics) and transferred to the processing vessel which had been temporarily inverted. Prior to closing the processing vessel, a portion of the first processing (extracting) solution was gently added to the processing vessel and th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| processing time | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com