Pharmaceutical Composition Comprising An Antigen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
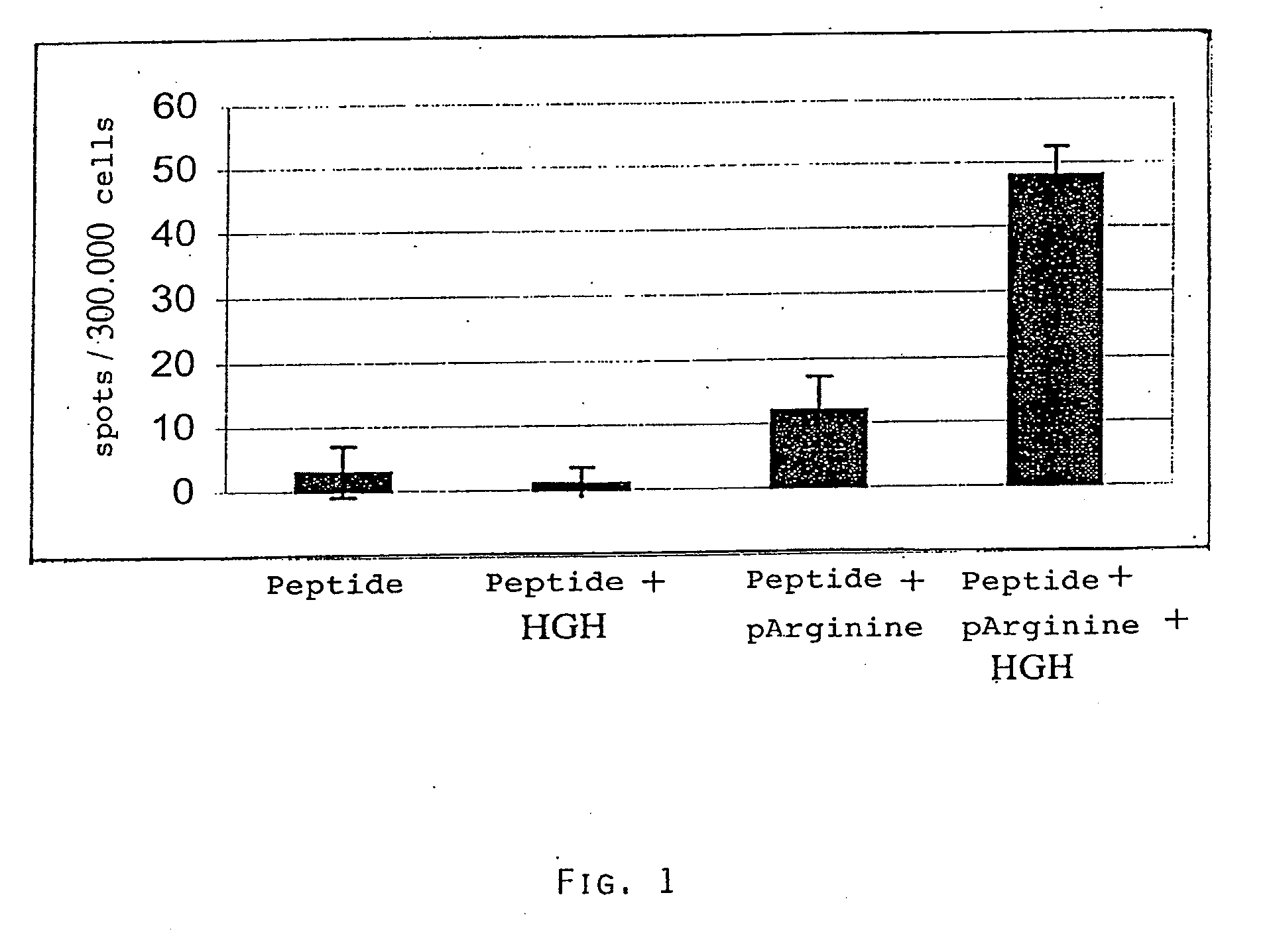
Induction of Antigen Specific T Cells is Greatly Enhanced by Coinjection of Combination of Poly-L-Arginine and Human Growth Hormone
[0043]
MiceC57BL / 6 (Harlan / Olac)PeptideVYDFFVWL derived from mousetyrosinase related protein-2.Restricted to H-2Kb (Bloom et al.,1997).Dose: 100 μg / mouse.Control peptideSIINFEKL derived from ovalbumin.Restricted to H-2Kb (Carbone andBevan, 1989).Poly-L-arginine 60 (pR60)poly-L-arginine with an averagedegree of polymerization of 60arginine residues; SIGMA chemicalsDose: 100 μg / mouseHuman Growth Hormone (HGH)0.02 IU / injectionSAIZEN, Laboratoires Serono)
[0044]Peptides were synthesized by standard solid phase F-moc synthesis, HPLC purified and analysed by mass spectroscopy for purity.
Experimental Groups (5 Mice Each)
[0045]1) TRP-2 peptide
2) TRP-2 peptide+HGH
3) TRP-2 peptide+pR 60
4) TRP-2 peptide+pR 60+HGH
[0046]On day 0 mice were injected subcutaneously with a total volume of 100 μl containing the above mentioned compounds. Animals were sacrificed 10 days afte...
example 2
Preferred Antigens to be Used for Providing a Vaccine Composition According to the Present Invention
[0048]1. HCV: antigens according to table 1[0049]and the antigens disclosed in Lamonaca et al.,[0050]Hepatology 30(4) (1999), 1088-1098.
Hepatitis C Peptides
[0051]
TABLE 1CD4CD8epitopesSequenceReferencesepitopesSequencesReferencesCoreKFPGGGQIVGGVYL(Hoffmann et al., 1995)CoreDLMGYIPAV(Sarobe et al., 1998)23-44LPRRGPRL132-140(SEQ ID NO:3)(SEQ ID NO:1)E2 / NS1——E2 / NS1FLLLADARV(Wentworth et al., 1996)723-731(SEQ ID NO:17)NS3GYKVLVLNPSVAAT(Diepolder et al., 1997)NS3CINGVCWTV(He et al., 1999;1248-1261(SEQ ID NO:6)1073-1081(SEQ ID NO:4)Rehermann et al., 1996)CoreADLMGYIPLVGAPL(Hoffmann et al., 1995)131-150GGAARA(SEQ ID NO:2)Notes:Core 23-44 contains two CD8 epitopes: core 31-40 (VGGVYLLPRR) (SEQ ID NO:18) and core 35-44 (YLLPRRGPRL) (SEQ ID NO:19) (Battegay et al., 1995; Rehermann et al., 1996)Core 131-150 contains the CD8 epitope core 132-140 (DLMGYIPLV) (SEQ ID NO:20) (Wentworth et al., 1996)
2...
example 3
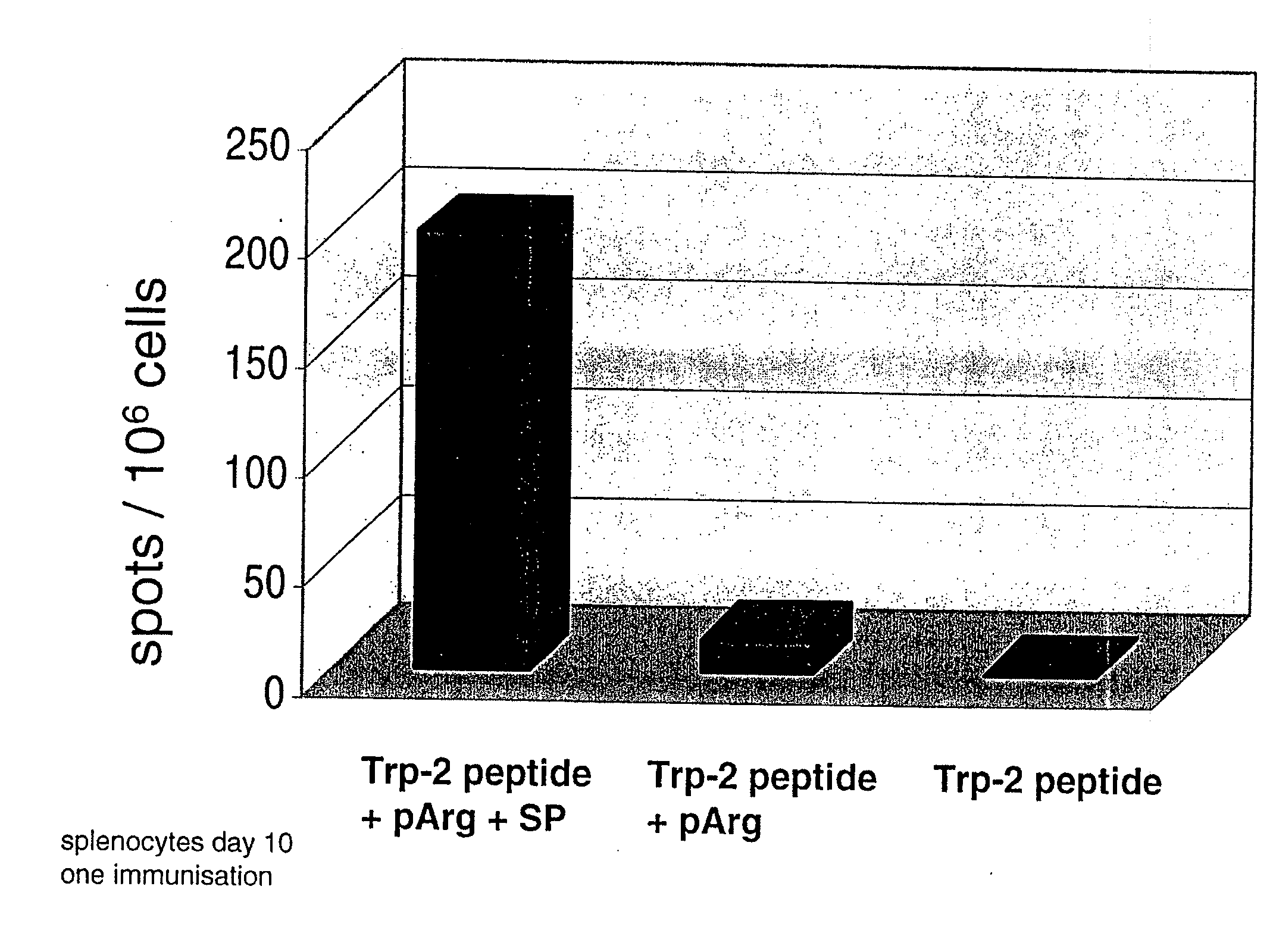
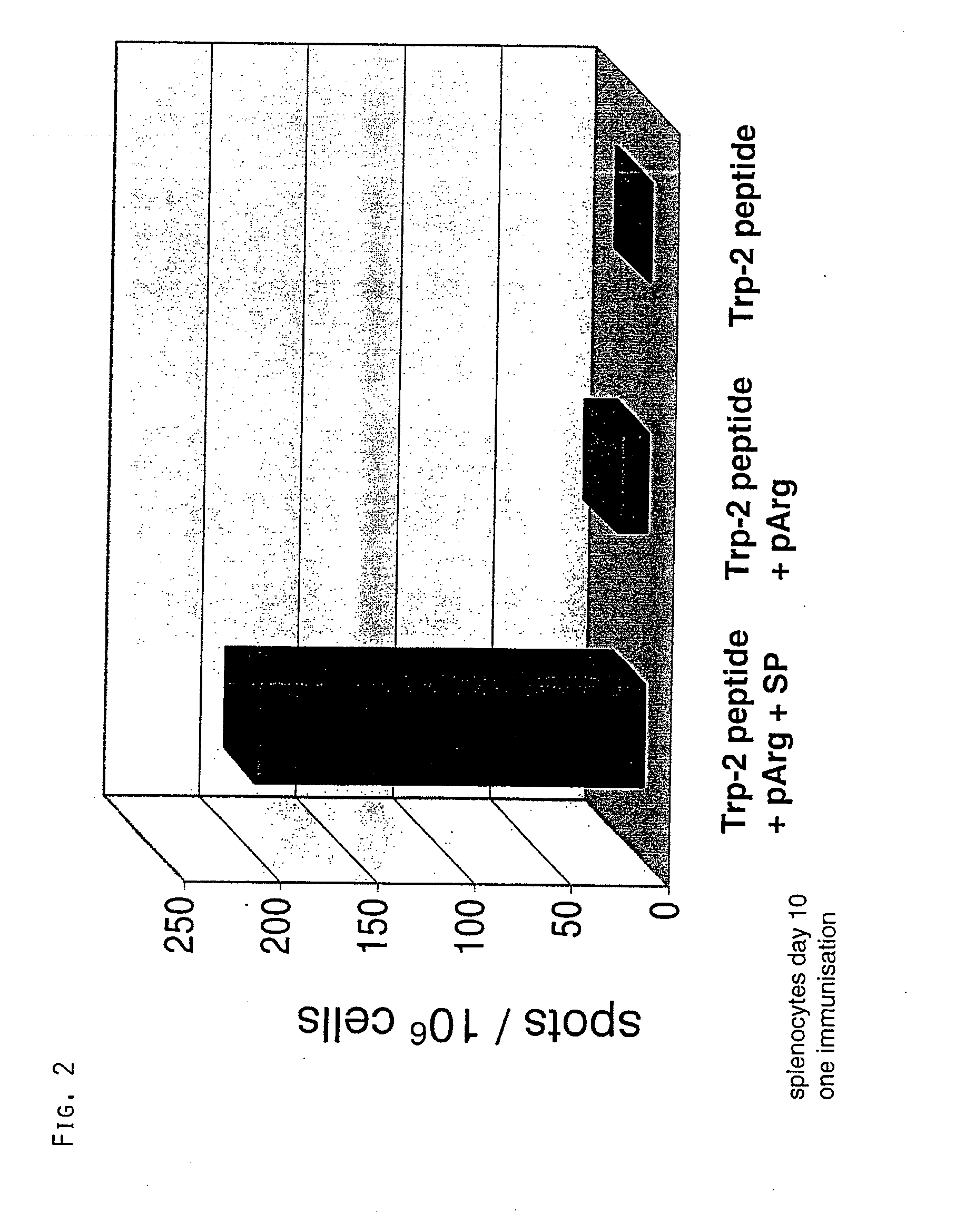
Co-Injection of Substance P and pArg
[0053]Substance P was tested as a further example for a neuroactive peptide (Marx, Science 205 (1979), 886). Substance P (RPKPQQFFGLM-NH2; SP) was synthesized and purified according to standard procedures. Experiments were conducted as in Example 1 with the exception that spleens instead of lymph nodes were used.
[0054]Splenocytes were prepared from spleens as follows: cells were passed through a 70 μm sieve and washed once with DMEM medium (GIBCO BRL). Red blood cells were lysed with “red blood cells lysis buffer” (Sigma) for 1 minute and washed twice with DMEM medium (GIBCO BRL). Cells were adjusted to 3*106 cells / ml in complete medium (DEMEM+5% FCS). IFN-γ-ELISPOT was carried out as described (Miyahira et al., 1995).
Experimental Groups (2 Mice Each)
[0055]1) TRP-2 peptide
2) TRP-2 peptide+pR 60
3) TRP-2 peptide+pR 60+substance P
Per injection 10 nmoles SP have been administered to mice.
[0056]The results are depicted in FIG. 2. It is demonstrated wit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Composition | aaaaa | aaaaa |
| Immunogenicity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com