Compositions and Methods for Generation of Infectious Hepatitis C Virus in Immortalized Human Hepatocytes
a technology of hepatitis c virus and human hepatocytes, which is applied in the field of composition and methods of propagating the infectious hepatitis c virus genome and infectious particles, can solve the problems of difficult progress in understanding the biology of hcv, and achieve the effect of higher magnification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Replication of HCV Genome and Virus Protein Expression
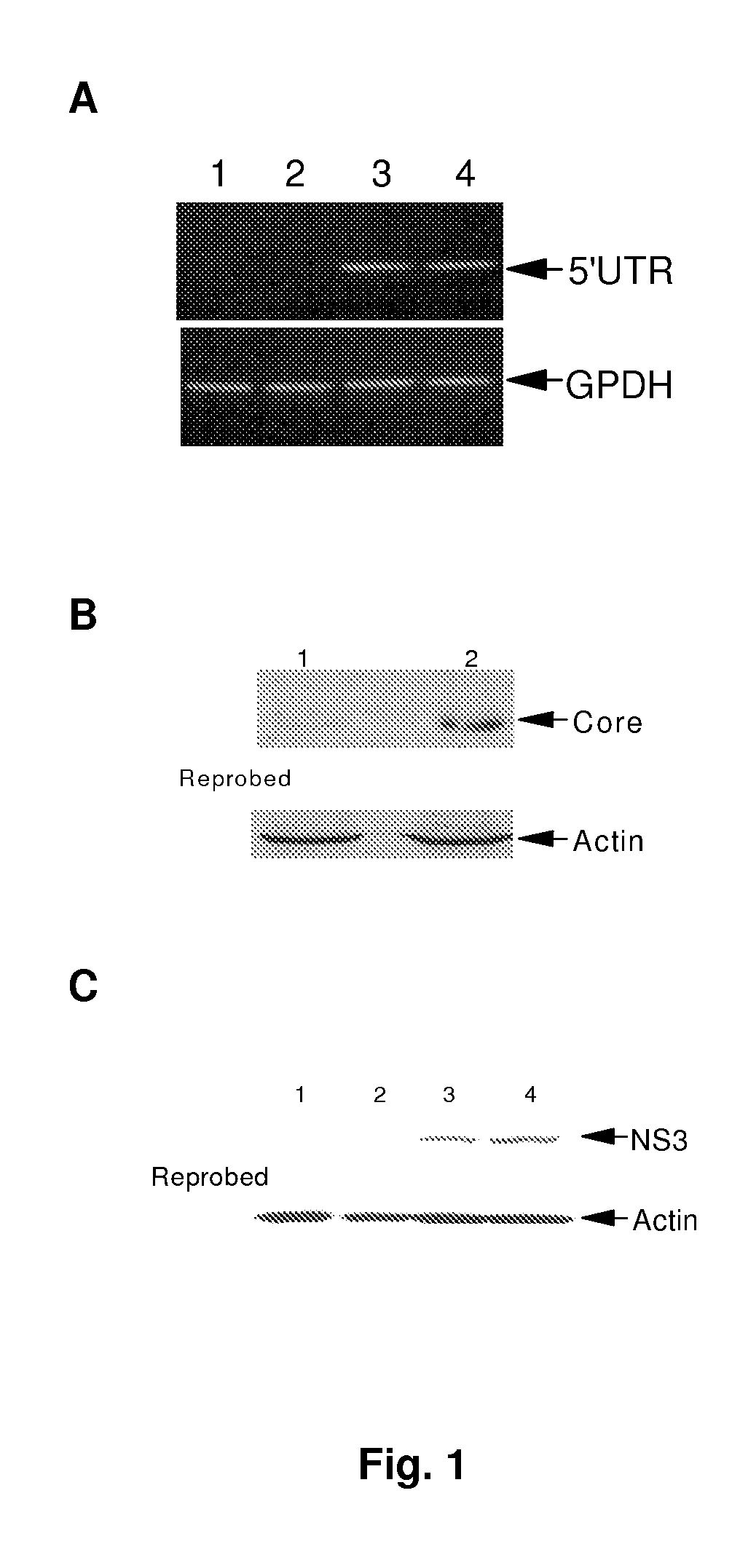
[0036]The object of the invention is a cell line to provide HCV 1a genotype replication and generation of infectious virus particles. The inventors have previously described the generation of immortalized human hepatocytes (IHH) by transfection of HCV core genomic region from genotype 1a, as well as the conditions and requirements for maintaining IHH in culture (2, 25) which are herein incorporated by reference. Full-length RNAs from HCV genotype 1a (clone H77, GenBank Accession number NC—004102, SEQ ID NO:1) has also previously described (3, 13, 14) and are herein incorporated by reference. Restriction enzyme Xba I and T7 RNA polymerase were obtained from Invitrogen and Promega, (Madison, Wis.) respectively and used according to the supplier's protocol. The individual elements of the inventors' methodology is generally well known and described in detail in numerous laboratory protocols, one of which is Molecular Cloning 2nd edit...
example 2
Immunogold Localization of Virus-Like Particles
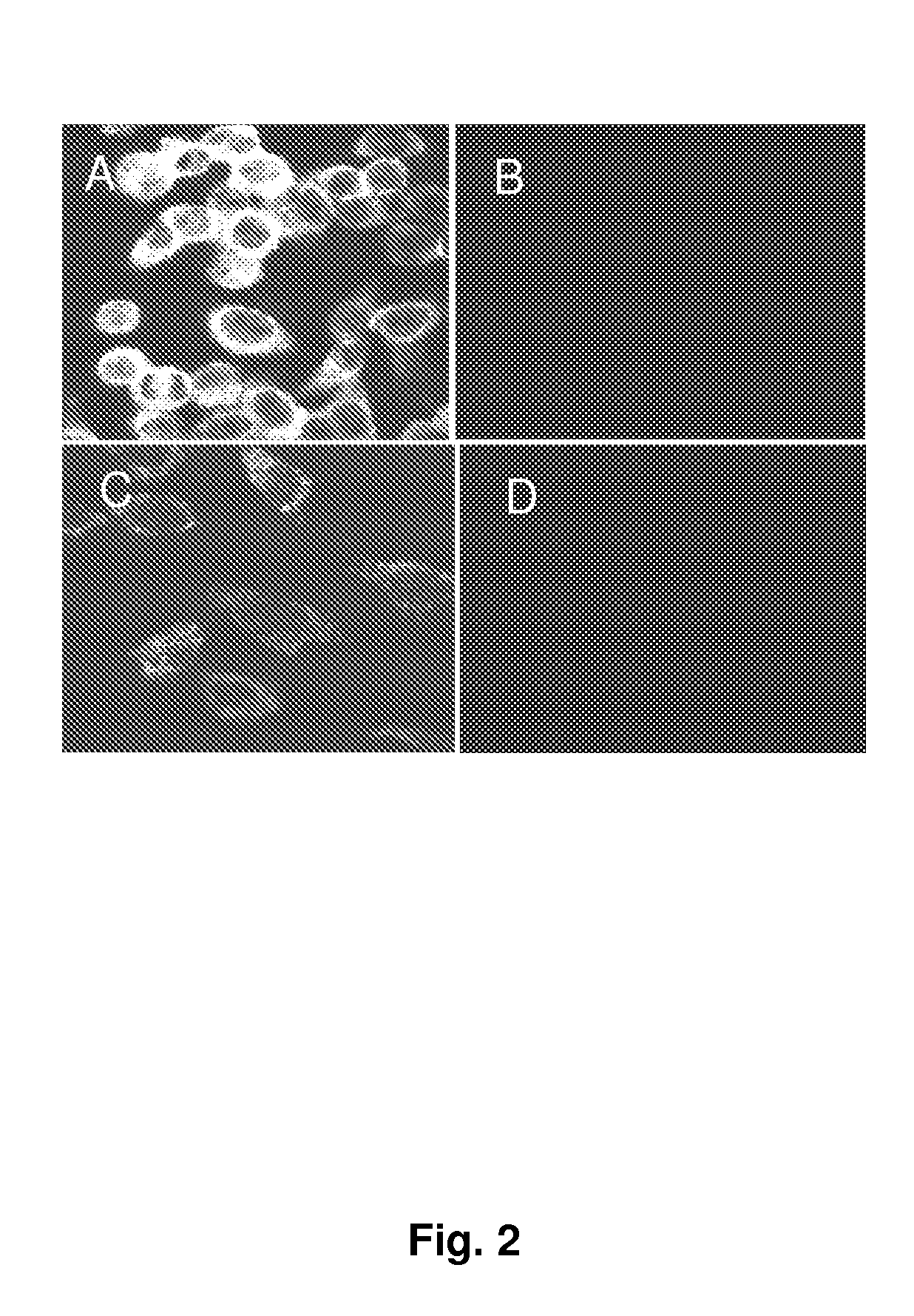
[0041]Phase contrast microscopy suggested that HCV genome transfected IHH were swollen with large vacuoles in the cytoplasm, whereas negative controls did not show any detectable changes. The inventors also examined cellular changes using an electron microscopy, and found at the ultrastructural level that some of these vacuoles appeared to be empty (FIG. 3, panels A and E). Others vacuoles contained lipid (FIG. 3, panel E) or material isolated for degradation (FIG. 3, panel D). Ultrastructural changes also included an increased polymorphism of nuclei (FIG. 3, panel E). Immunogold labeling was performed for localization of HCV like particles in transfected IHH. For this, transfected IHH (4 days in culture) were detached from collagen coated petridishes by a brief trypsin treatment, pelleted in a microcentrifuge, and fixed in 4% paraformaldehyde and 1% glutaraldehyde in PBS for 16 h at 4° C. After washing with PBS, cells were further wash...
example 3
Infection of IHH by HCV from Culture Medium
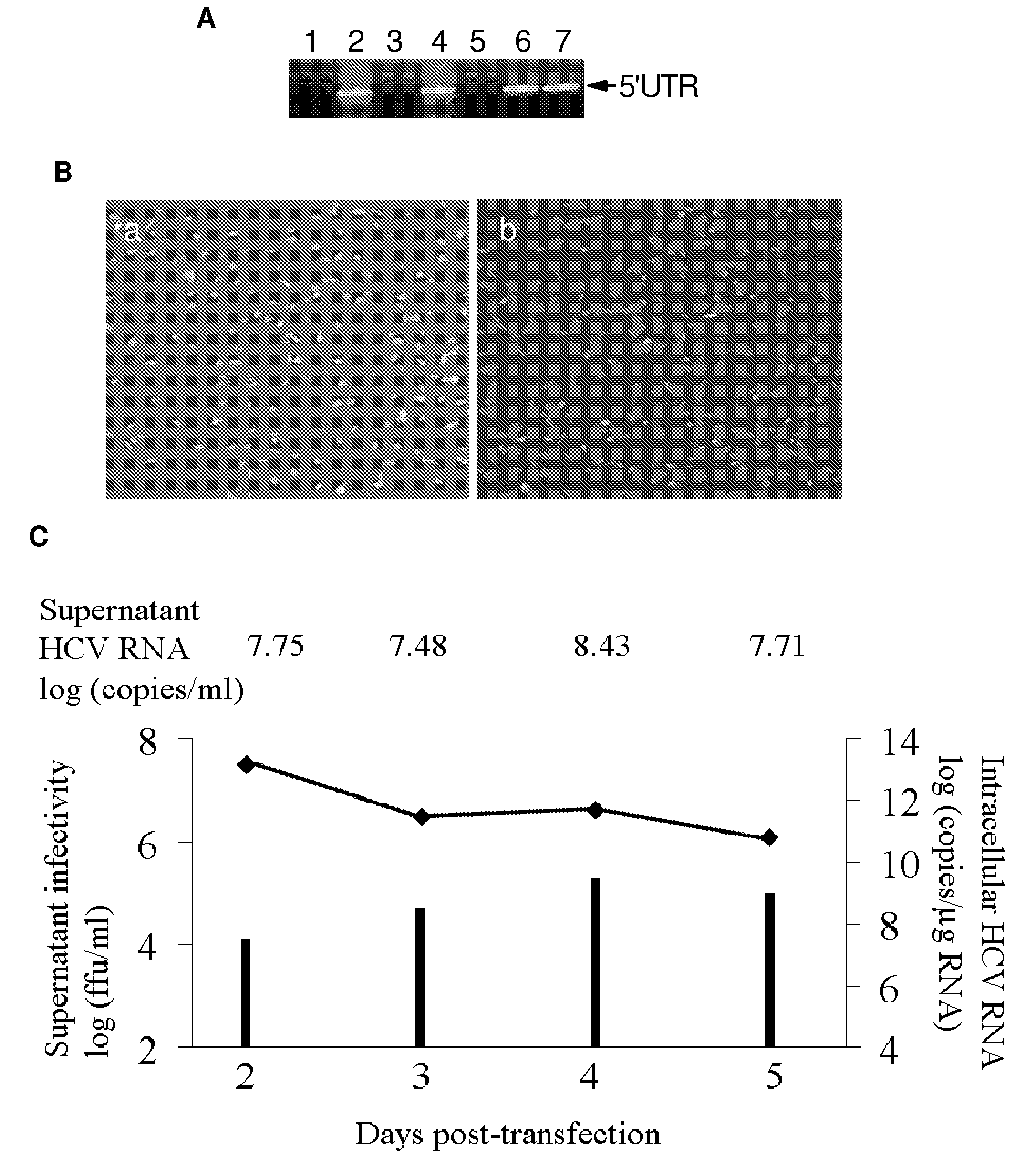
[0043]Inventors next examined the presence of HCV in cell culture medium from IHH. After different transfection time periods, culture medium was filtered through a 0.45 Mm cellulose acetate membrane (Millipore, Bedford, Mass.), and concentrated to 10-20 fold by Millipore ultrafiltration (100 kDa cut off) and used for detection of HCV genomic sequence by RT-PCR (FIG. 4, panel A). The presence of HCV 5′ UTR was detected in culture medium from HCV genome transfected IHH, but not from polymerase defective HCV RNA transfected IHH. The inventors obtained ˜1.1×108 genome copies / ml of culture medium using real-time RT-PCR, as recently described (32). Culture supernatant collected for 7 days suggested a peak of HCV genome copy number between 4 and 5 days after transfection.
[0044]The inventors then determined whether the culture medium contained infectious HCV. For this, culture medium was serially diluted two fold and inoculated into naive IHH. Cell...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com