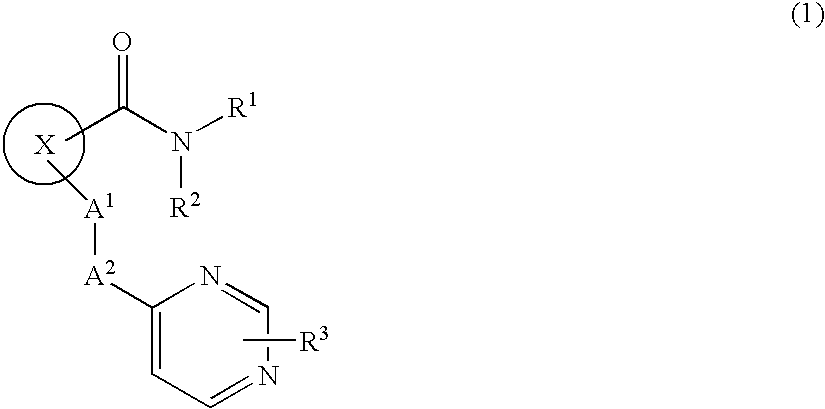
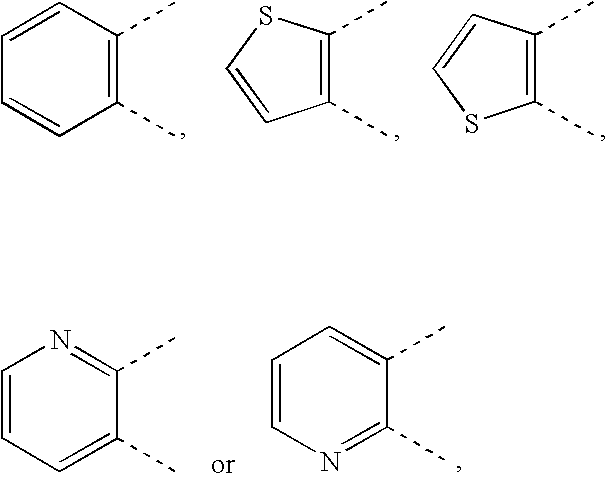
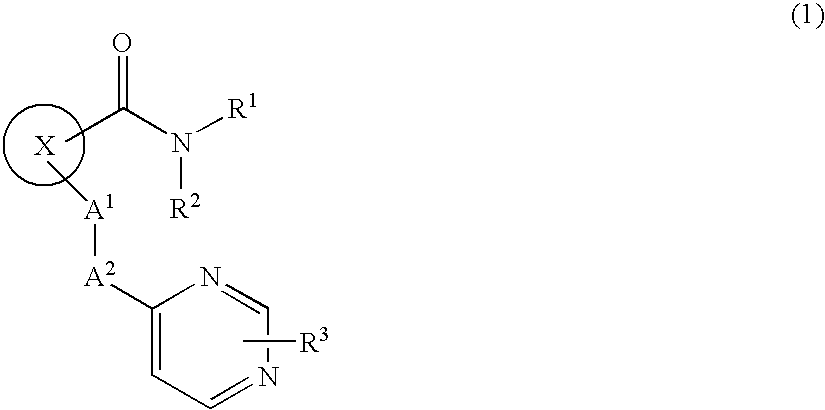
Novel Cyclic Compound Having Pyrimidinylalkylthio Group
a technology of pyrimidinylalkylthio group and cyclic compound, which is applied in the direction of biocide, drug composition, cardiovascular disorder, etc., can solve the problem of no description of cyclic compound having a pyrimidinylalkylthio group, and achieve excellent antiangiogenic
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
production example
Reference Example 1
2-Dimethylamino-4-trifluoromethylpyrimidine (Reference Compound No. 1-1)
[0148]
[0149]2-Chloro-4-trifluoromethylpyrimidine (600 μL, 5.0 mmol) was dissolved with 2.0M dimethylamine in methanol (10 mL), and stirred for 2 hours in a sealed tube at 60° C. The reaction mixture was diluted with ethyl acetate (50 mL), and the whole was washed with water (50 mL) and dried over anhydrous magnesium sulfate. The solvent was evaporated under reduced pressure to give 640 mg of the title Reference Compound as a colorless oil (Yield: 0.67%).
[0150]1H-NMR (400 MHz, CDCl3)
[0151]δ 3.22 (s, 6H), 6.72 (d, J=4.9 Hz, 1H), 8.48 (d, J=4.9 Hz, 1H)
reference example 2
2-Dimethylamino-4-methoxycarbonylpyrimidine (Reference Compound No. 2-1)
[0152]
[0153]2-Dimethylamino-4-trifluoromethylpyrimidine (320 mg, 1.7 mmol, Reference Compound No. 1-1) and sodium hydroxide (670 mg, 17 mmol) were suspended in a mixed solvent of methanol (5.0 mL) and water (5.0 mL), and then the mixture was stirred while radiated with microwave for 2 and a half hours at 160° C. in a sealed tube. The reaction mixture was diluted with ethyl acetate (30 mL), and extracted with water (30 mL), and then extracted with saturated aqueous sodium hydrogencarbonate solution (30 mL). The extracted aqueous layer was adjusted to pH7 with 6M hydrochloric acid, and was concentrated under reduced pressure. The remaining residue was suspended in N,N-dimethylformamide (5.0 mL), then sodium hydrogencarbonate solution (1.3 g, 14 mmol) and methyl iodide (0.87 mL, 17 mmol) were added thereto, and the whole was stirred for 23 hours at room temperature. The reaction mixture was diluted with ethyl aceta...
reference example 3
2-Amino-4-methoxycarbonylpyrimidine (Reference Compound No. 3-1)
[0156]
[0157]Triethylamine (29 mL, 210 mmol) was added to a suspension of methyl 4-butoxy-2-oxo-3-butenate (37 g, 200 mmol, JP-A-2003-89690) and guanidine hydrochloride (23 g, 240 mmol) in propionitrile (50 mL), and the mixture was stirred for 4 hours at 100° C. The reaction mixture was concentrated under reduced pressure, and the residue was purified by silica gel column chromatography to give 18 g as the mixture of the title Reference Compound and the butyl ester form of the title Reference compound as a gray-white solid (Yield: 60%).
[0158]1H-NMR (500 MHz, DMSO-d6)
[0159]δ 3.85 (s, 3H), 6.99-7.06 (m, 3H), 8.48 (d, J=4.8 Hz, 1H)
[0160]As described below, Reference Compounds Nos. 3-2 to 3-4 were obtained following the method similar to that of Reference Compound No. 3-1, using the corresponding compounds selected from compounds which are on the market or compounds which are commonly known.
4-Methoxycarbonyl-2-methylthiopyri...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com