Methods useful for the treatment of pain, arthritic conditions or inflammation associated with a chronic condition
a technology of chronic conditions and pain, applied in the direction of heterocyclic compound active ingredients, biocide, drug compositions, etc., can solve the problems of oxycodone marketed versions, limited use, dependence and abuse,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Evaluation of the Efficacy and Safety of an Oral Opioid Pharmaceutical Composition
[0268]A clinical trial of an exemplary oral opioid pharmaceutical composition was conducted as follows. The “study drug” used in the clinical trial is a controlled release oral formulation containing oxycodone as the API. The clinical trial was conducted in subjects with moderate to severe chronic pain due to osteoarthritis of the hip or knee. A primary objective of the clinical trial was to study the efficacy and safety of the study drug in these subjects. A secondary objective of the clinical trial was to compare quality of life measures in these subjects with moderate to severe chronic pain due to osteoarthritis of the hip or knee who receive the study drug as compared with those who receive placebo.
[0269]For this clinical trial, a multicenter, randomized, double-blind, placebo-controlled, phase III study was conducted in approximately four hundred subjects with moderate to severe chronic pain due t...
example 2
Preparation of Opioid Formulations
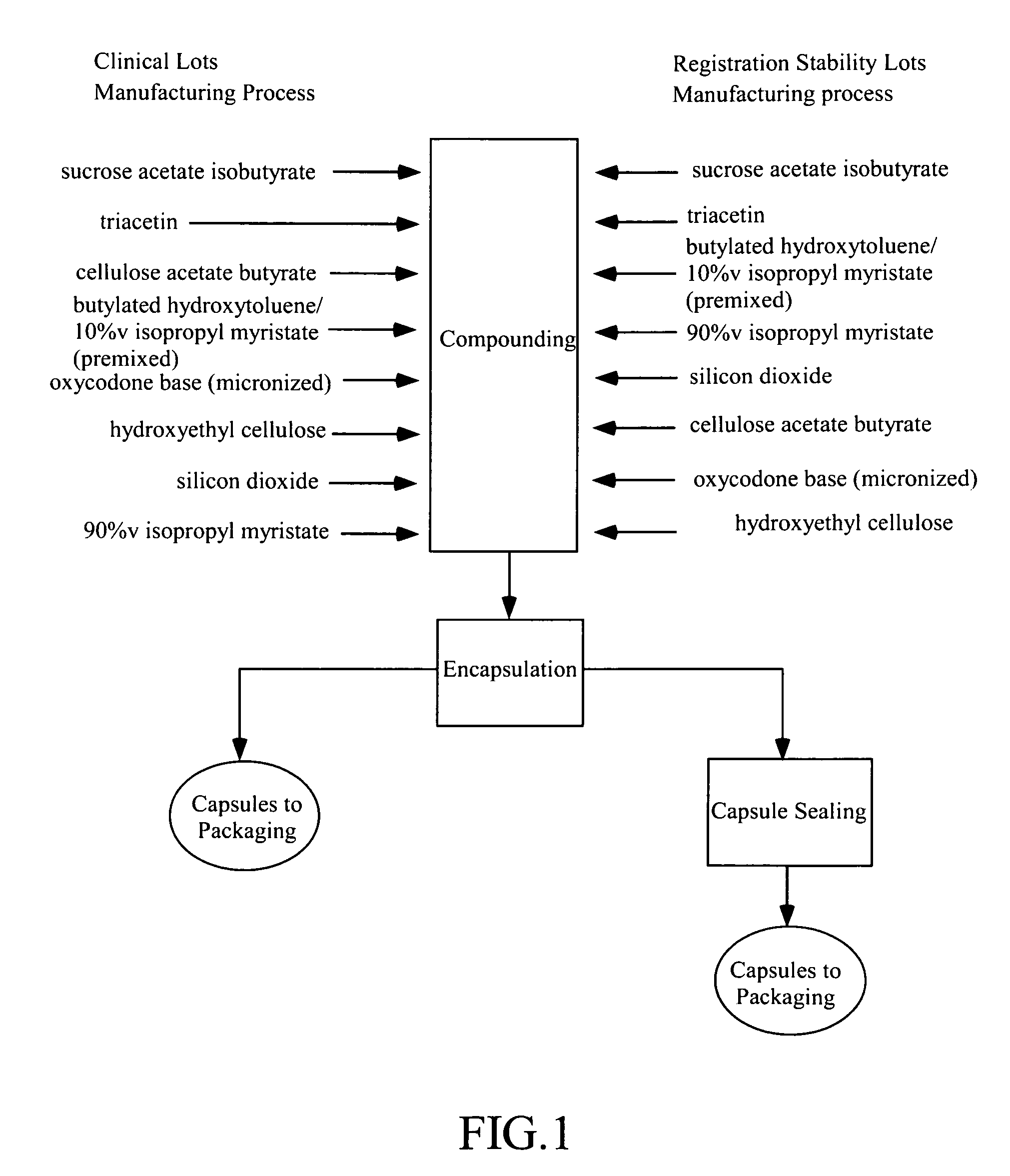
[0532]Exemplary opioid dosage forms comprising oxycodone are prepared as described herein. For clinical studies as described in Example 1, capsules having different amounts of oxycodone are produced.
[0533]Capsule formulations containing oxycodone at various dose levels (5.0, 10.0, 20.0, 30.0 and 40.0 mg / capsule) and matching placebo capsules are prepared.
[0534]The components, pharmaceutical grade, and function of each component used to make oxycodone capsules are provided in Table 16 below.
TABLE 16Components for Oxycodone CapsulesComponentFunctionOxycodone base (micronized)Active pharmaceutical ingredientSucrose acetate isobutyrateLiquid carrier materialTriacetin, USPSolventIsopropyl myristate, NFRheology modifierCellulose acetate butyrate, NF / EP,Network formerethanol washed (grade 381-20 BP)Hydroxyethyl cellulose, NFHydrophilic agentColloidal silicon dioxide, NFViscosity enhancing agentButylated hydroxytoluene, NFAntioxidantHard gelatin capsuleDosa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com