Method of measuring blood component and sensor used in the method
a blood component and sensor technology, applied in the field of method of measuring a blood component and a sensor used in the method, can solve the problem of requiring a laborious correction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0040]Hereinafter, examples of the present invention will be described along with a comparative example.
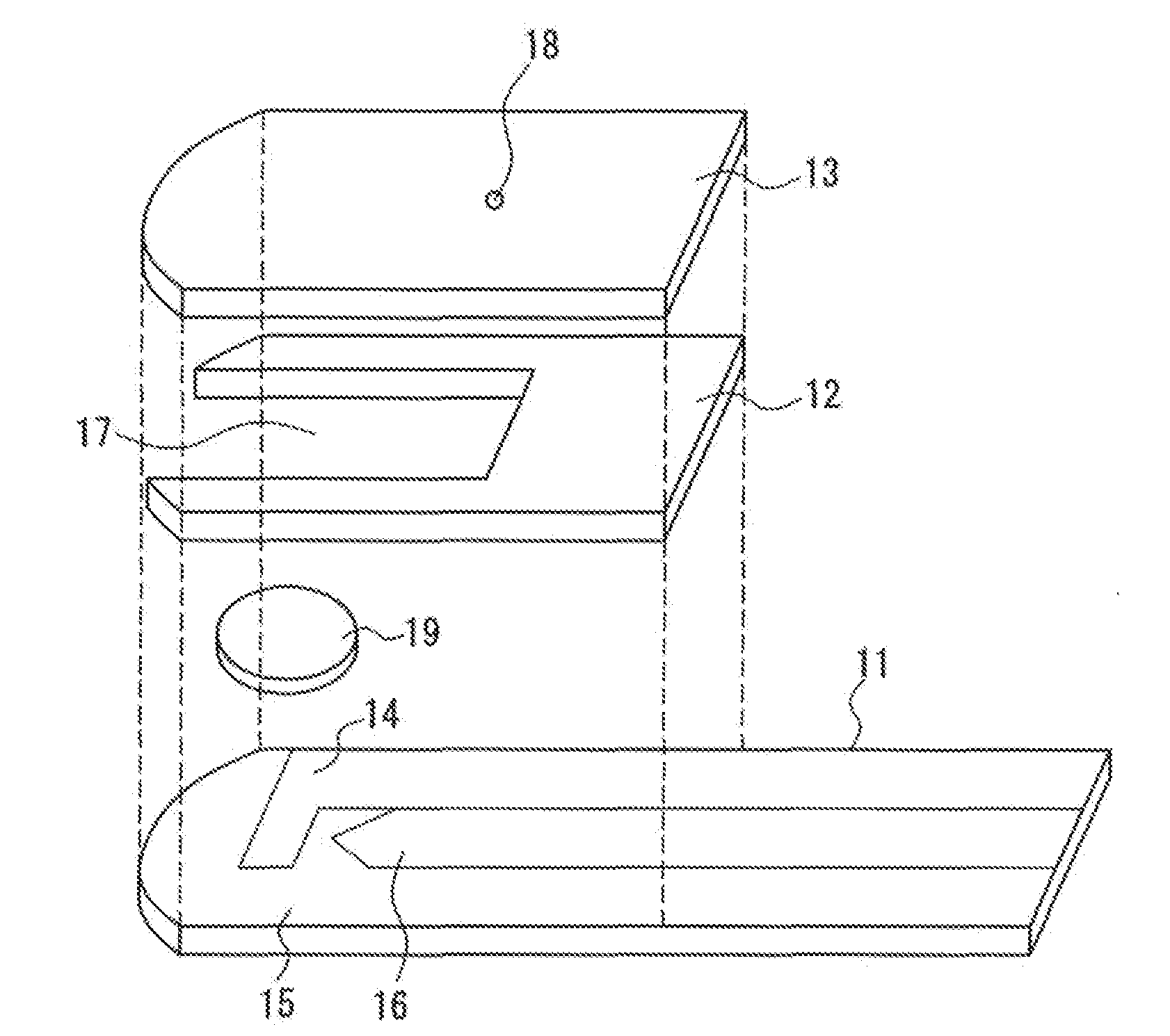
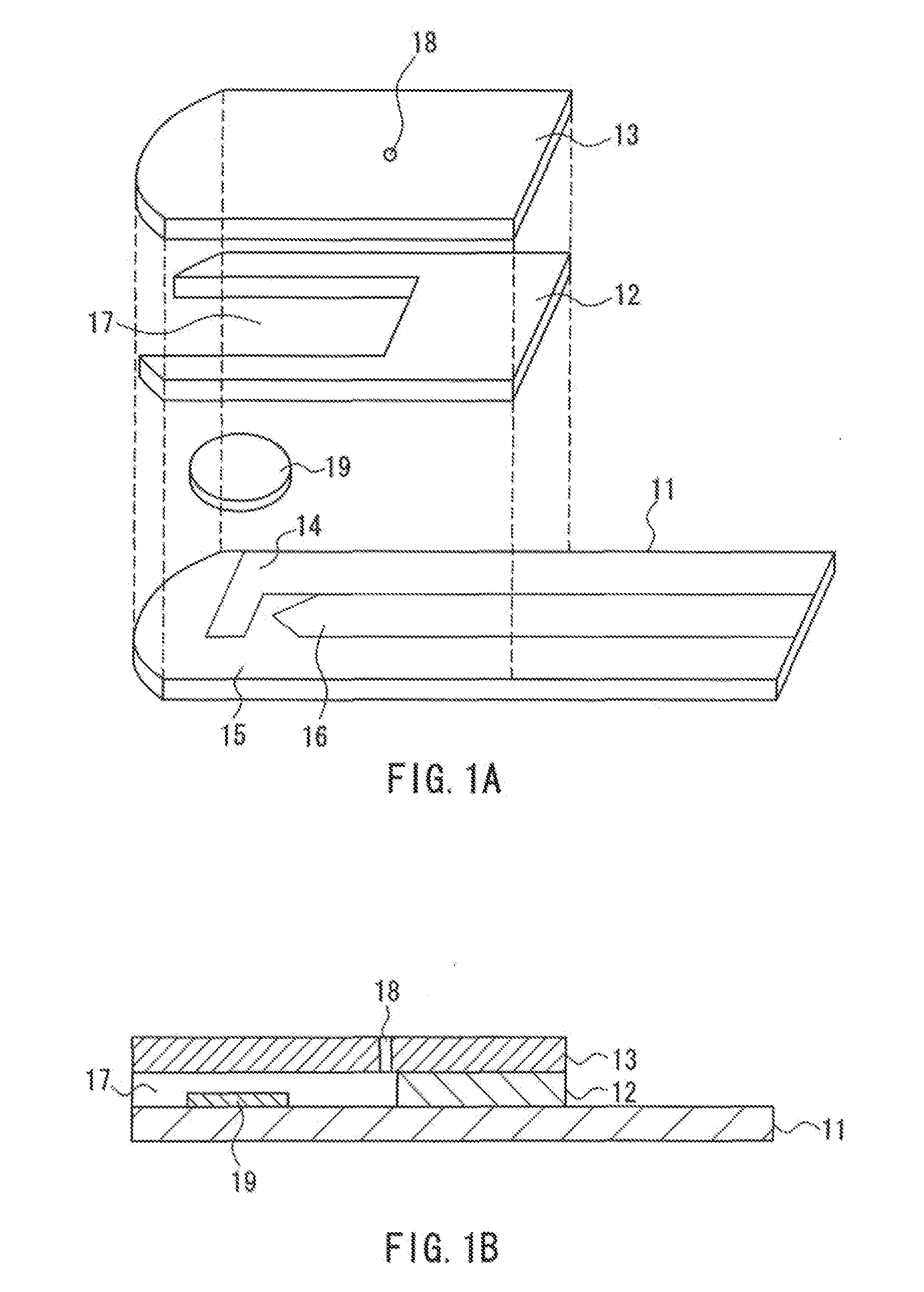
[0041]Sensors having the configuration as shown in FIG. 1 were produced in the manner described above. A reagent solution having the following composition was prepared, which was dropped on an analysis portion of each sensor and then dried to form a reagent portion.
(Composition of Reagent Portion)
[0042]enzyme (PQQ-GDH)
[0043]mediator (potassium ferricyanide)
[0044]hydrophilic polymer (CMC)
[0045]enzyme stabilizer (maltitol)
[0046]crystal homogenizing agent (taurine)
[0047]membrane protein solubilizer (sodium cholate: 1.2 mM)
[0048]On the other hand, from two types of human whole blood with glucose concentrations of 100 mg / dL and 400 mg / dL, six types of human whole blood samples were prepared by adjusting the Hct to 25%, 45%, and 65%.
[0049]With regard to each sample, the measurement was carried out in the following manner. The sensor was set in a dedicated measuring device (a meter), and...
example 2
[0052]Sensors were produced in the same manner as in Example 1. In these sensors, the reagent portion contained the same components as those in Example 1, but the amount of sodium cholate as the membrane protein solubilizer was changed. More specifically, in the present example, three types of sensors, namely, the sensor with 0.8 mM of sodium cholate, the sensor with 1.8 mM of sodium cholate, and the conventional sensor without the membrane protein solubilizer were produced.
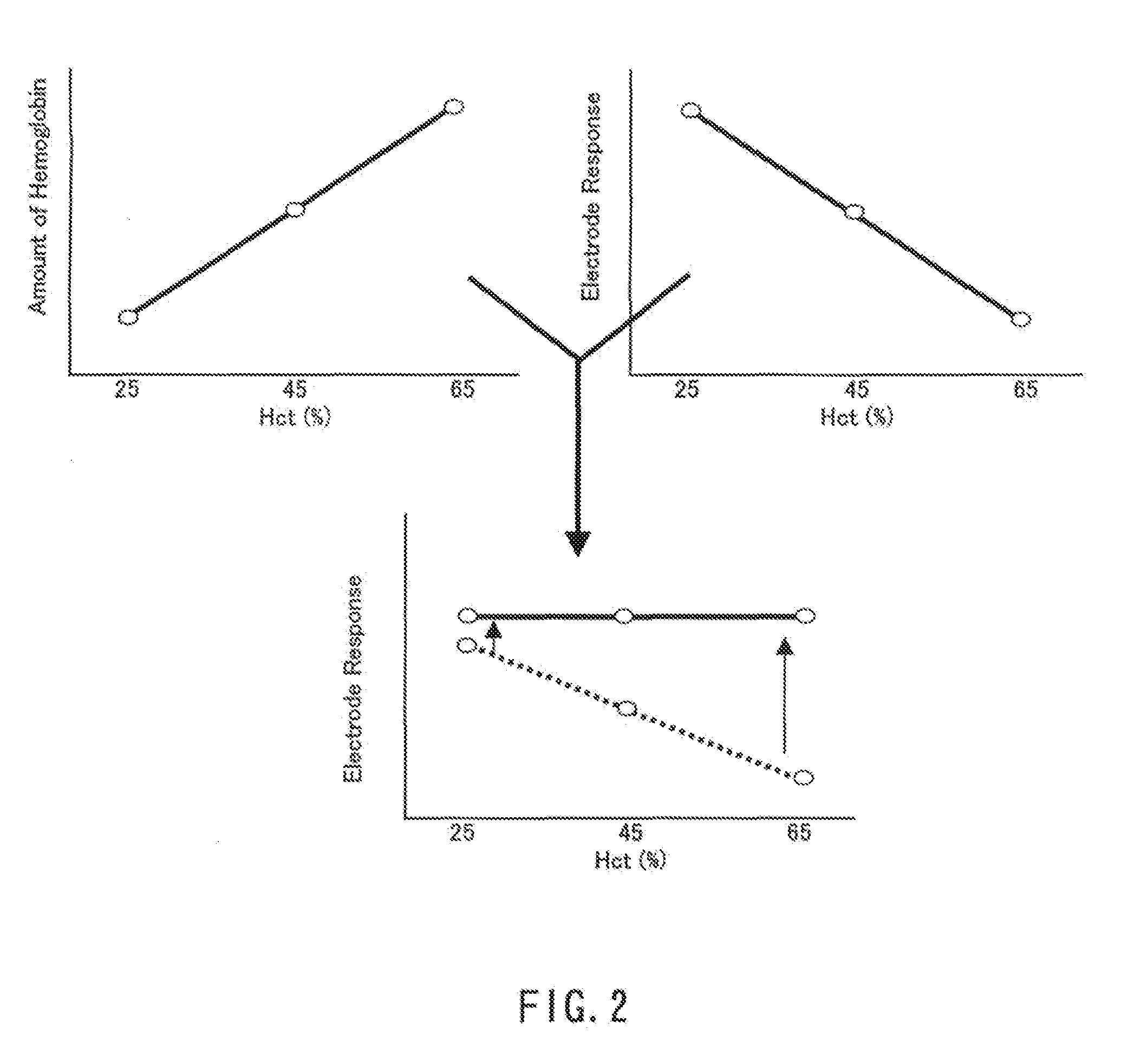
[0053]The measurement was carried out in the same manner as in Example 1. The conditions for the current measurement and the number (n) of times the measurement was performed also were the same as those in Example 1. FIG. 5 shows the results of the measurement performed with regard to three types of human whole blood samples prepared by adjusting the Hct of human whole blood with glucose concentration of 100 mg / dL to 25%, 45%, and 65%. In the graph of FIG. 5, the detected current with regard to the sample with th...
example 3
[0055]Sensors were produced in the same manner as in Example 1. In these sensors, the composition of the reagent portion was the same as in Example 1 except that the type of the membrane protein solubilizer was changed. More specifically, in the present example, three types of sensors respectively employing the following membrane protein solubilizers were produced.
(Membrane Protein Solubilizer)
[0056]sodium taurocholate (1.2 mM)
[0057]sodium taurodeoxycholate (1.2 mM)
[0058]sodium glycocholate (1.2 mM)
[0059]Using these sensors, the measurement was performed in the same manner as in Example 1 with regard to six types of human whole blood samples prepared by adjusting the Hct of two types of human whole blood with glucose concentrations of 100 mg / dL and 400 mg / dL to 25%, 45%, and 65%. FIG. 6 shows the result of the measurement using the sensor employing sodium taurocholate as the membrane protein solubilizer, FIG. 7 shows the result of the measurement using the sensor employing sodium ta...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com