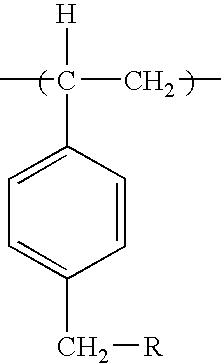
Biocidal particles of methylated polystyrene
a technology of methylated polystyrene and biocidal particles, which is applied in the field of biocidal particles of methylated polystyrene, can solve the problems of difficult preparation of poly-1,3-dichloro-5-methyl-5-(4′-vinylphenyl)hydantoin as uniform particles, and noticeable chlorine odor, so as to achieve reasonable biocidal efficacy, less chlorine outgassing, and less steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
A Representative Preparation of Chlorinated Methylated Polystyrene Hydantoin Beads
[0037]Porous beads of 5.6% crosslinked chloromethylated polystyrene (containing 20.85% by weight chlorine) obtained from Suqing Group (Jiangyin, Jiangsu, PRC) having particle sizes in the range 180 to 425 μm, but undetermined pore sizes, were cleaned by soaking them in acetone (400 mg / mL) for 30 minutes at 25° C. and then by passing 3 portions of acetone (0.5 mL per g) through them in a filter funnel. Following drying to constant weight under vacuum at 50° C., 20.3 g (about 0.12 mole of active chlorine) of the beads were suspended in 150 mL of anhydrous DMF in a 250 mL flask fitted with a condenser. Then 16.5 g of anhydrous potassium carbonate (0.12 mole) and 15.4 g (0.12 mole) of 5,5-dimethylhydantoin were added, and the mixture was stirred for 72 hours at 95° C. After cooling the mixture to 25° C., suction filtration was used to isolate the beads. The beads were then soaked in 500 mL of boiling water...
example 2
An Alternative Representative Preparation of Chlorinated Methylated Polystyrene Hydantoin Beads
[0039]The potassium salt of 5,5-dimethylhydantoin was prepared by reacting 25.6 grams (0.2 mole) of 5,5-dimethylhydantoin with 11.2 grams (0.2 mole) of potassium hydroxide in 100 mL of boiling ethanol with stirring. The ethanol and product water were removed under vacuum to obtain the white salt. The salt was added to 200 mL of anhydrous DMF and heated to 95° C. until all of the salt dissolved. Then 8.12 grams (about 0.048 mole of active chlorine) cleaned chloromethylated polystyrene beads were added and the mixture was heated with stirring at about 100° C. for 12 hours. The unreacted potassium salt of the hydantoin and the DMF were recycled for further use, and the beads functionalized with hydantoin groups were washed and dried under vacuum at 85° C. until constant weight as in Example 1. The weight of the beads prepared in this manner was 11.0 grams (35.5% by weight add-on). Chlorinatio...
example 3
Representative Preparation of Brominated Methylated Polystyrene Hydantoin Beads
[0040]Methylated polystyrene hydantoin beads (5.0 grams) prepared as described in Example 1 were suspended in a solution containing 40 mL of 10% sodium hypobromite and 40 mL of water. The pH was adjusted to 7.0 using 2 N acetic acid. The mixture was stirred for 1 hour at 25° C. The brominated beads were removed by filtration, washed with three 100 mL portions of water, and dried under vacuum until constant weight was obtained. The bromine content determined by sodium thiosulfate / iodometric titration was 8.2% by weight. An infrared spectrum of a small sample of the beads (crushed to a powder) in a KBr pellet exhibited prominent bands at 1714 and 1776 cm−1 consistent with the presence of a monobrominated hydantoin functional group.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com