Anaplerotic Therapy of Huntington Disease and Other Polyglutamine Diseases
a polyglutamine disease and anaphylaxis technology, applied in the field of treatment and the prevention of huntington disease and other polyglutamine diseases, can solve the problems of elusive effectiveness of pharmacotherapy for neurodegenerative diseases associated with impaired energy metabolism, especially polyglutamine diseases, and achieve the effects of treating and/or and preventing a polyglutamine diseas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
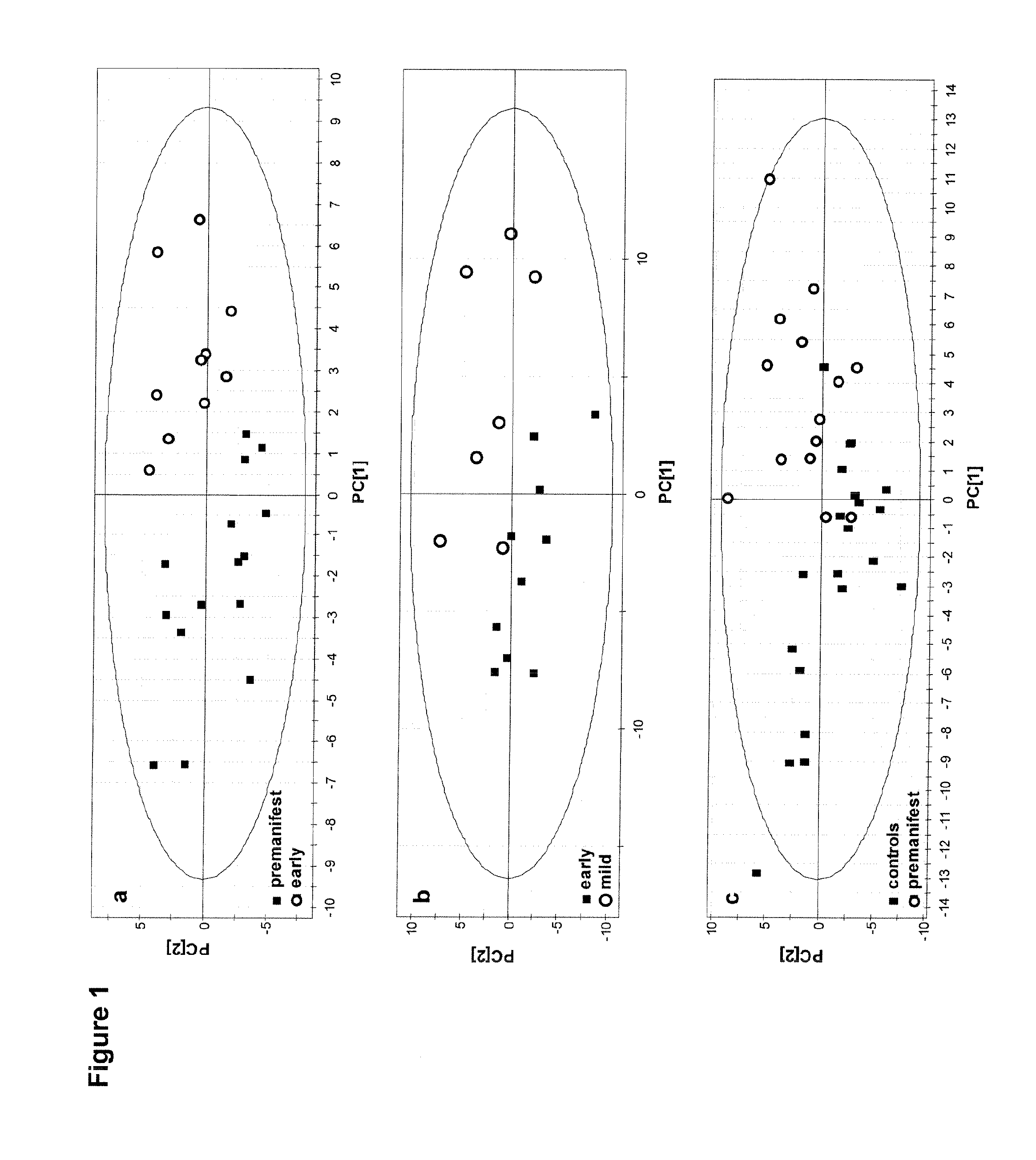
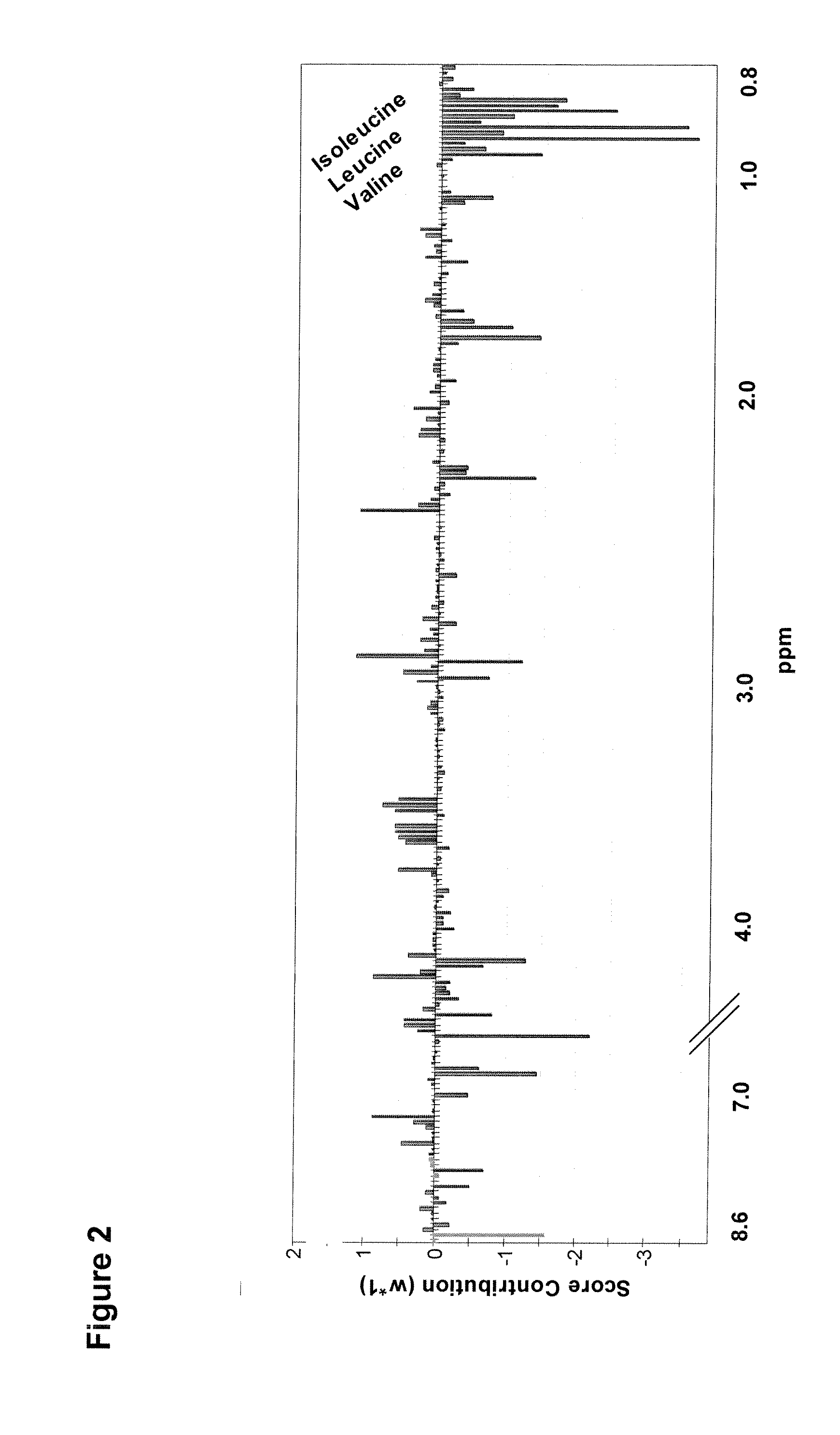
Identification of a Plasma Biomarker in Premanifest Carriers of Huntington Disease Indicating Early Energy Imbalance
[0037]Abbreviations: HD (Huntington disease), UHDRS (Unified Huntington disease rating scale), ppm (parts per million), PCA (principal components analysis), PLS (partial least square).
Abstract
[0038]Huntington disease (HD) is an autosomal dominant neurodegenerative disorder in which an energy deficiency is thought to play a role. Patients consistently lose weight, although the reason for this is unknown. In view of the specific access to premanifest carriers in HD, we performed a multiparametric study in a group of 32 individuals with no sign or little of the disease compared to 21 controls. Weight loss was observed even in premanifest carriers in the HD group, although their caloric intake was higher. Inflammatory processes were ruled out, as well as primary hormonal dysfunction, including ghrelin and leptin balance. Proton nuclear magnetic resonance spectroscopy on pl...
example 2
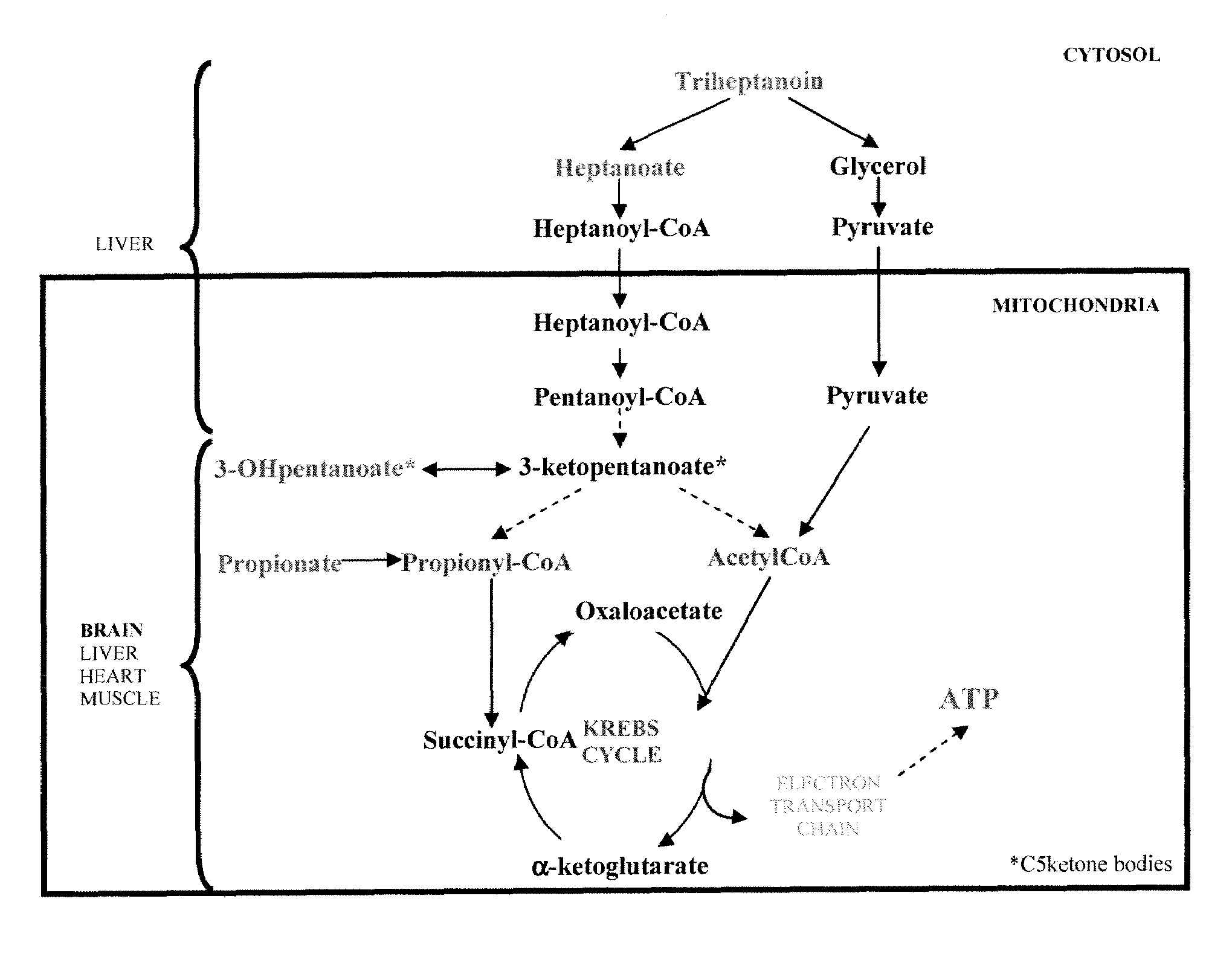
Triheptanoin Therapy of HD mouse models
Aims of the Study
[0096]A pilot study is conducted to test the effect of dietary triheptanoin therapy versus control diet on selected strains of HD R6 / 2 mice (Mangariani 1996, Cell 87: 493-506, Kosinski 1999, Neuroreport 10: 3891-6). The study includes (i) measuring rates of cerebral anaplerosis from heptanoate and brain ATP in R6 / 2 mice of different ages and in control mice in order to demonstrate the ability of triheptanoin metabolites to cross the blood brain barrier of R6 / 2 mice and to reverse central energy deficit; (ii) assessing, the therapeutic efficacy of triheptanoin by accurate behavioral testing, in vivo brain microdialysis (to assay neurotransmitters, triheptanoin metabolites, and BCAA) and neuropathological examination; (iii) metabolomic analyses on mouse plasma and urine.
Research Methods
[0097]R6 / 2 and control mice, on triheptanoin-enriched and control diets, are infused sequentially—at 4, 8 and 12 weeks—with various doses of [5,6,...
example 3
Therapy of Spinocerebellar Ataxia 7 (SCA7)
1 / In Vitro Trial
[0101]To create a simplified model of SCA7 in vitro we used primary cultures of dissociated cerebellar cells because lesion of the cerebellum, particularly the Purkinje cell (PC) layer, accounts for the ataxia phenotype in patients with SCA7. Our cerebellar cell cultures were composed of glial cells and neurons, 5 to 10% of which expressed calbindin (CaBP) identifying them as PC. To examine the effects of mutant ATXNT7 on PC survival, the cells were infected at DIV1 (1st Day In Vitro) with lentiviral vectors carrying truncated forms of normal and mutant ataxin 7 (ATXN7T: amino acids 1-232) fused to GFP (ATXN7T-10Q-GFP, ATXN7T-100Q-GFP). These lentiviral vectors allowed efficient expression of these proteins in about 90% of neurons, including Purkinje cells. Infection by ATXN7T-100Q-GFP led to massive neuronal loss, almost exclusively in Purkinje neurons (˜85% of Purkinje cell death versus ˜20% loss of other neurons), thus rep...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| resonance line widths | aaaaa | aaaaa |
| energy metabolism | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com