Acidothermus celluloyticus xylanase
a technology of xylanase and acidothermus, which is applied in the field of xylanases, can solve the problems of breaking down lignocellulose, being easily subjected to bacterial contamination, and the production of xylanase enzymes being thermally unstable,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Cloning of the Xyl-1
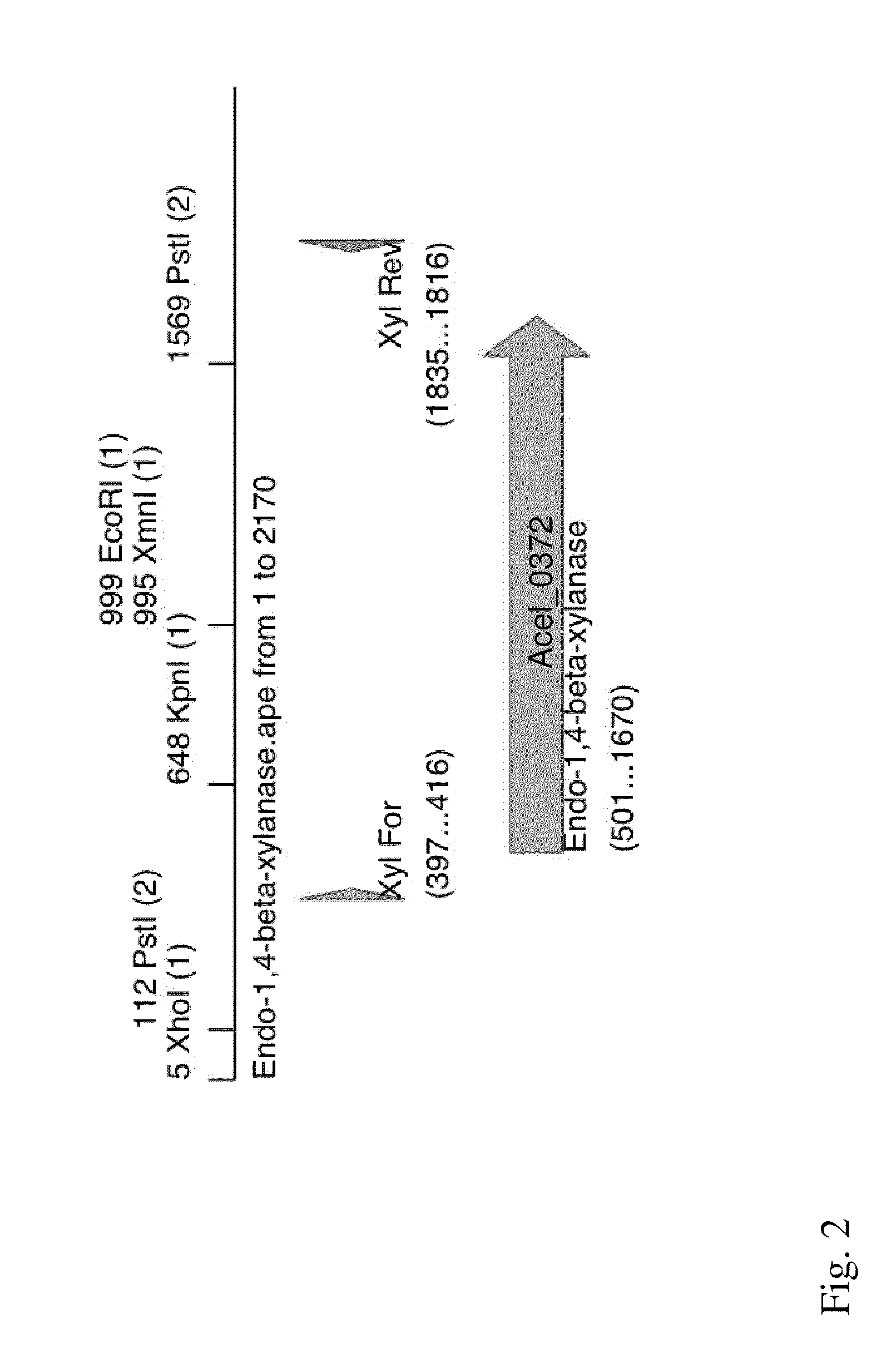
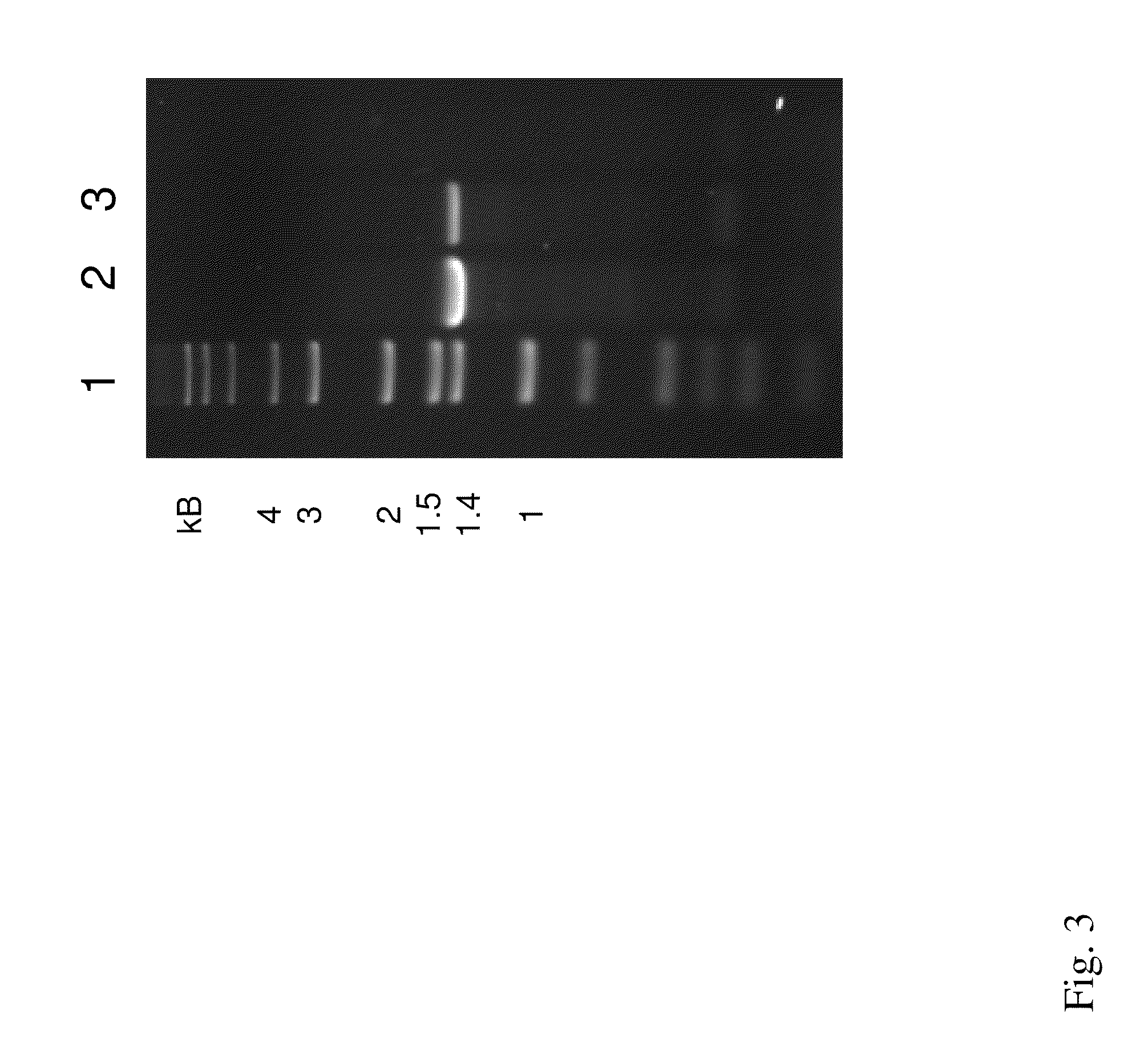
[0099]According to the A. cellulolyticus 11B genome annotation, Acel—0372 encodes a predicted secreted xylanase (Xyl-1). In order to clone Acel—0372, A. cellulolyticus 11B (ATCC 43068) genomic DNA was purified as previously described (15), and oligonucleotide primers were designed in order to PCR amplify the complete Acel—0372, the predicted 1.4-kb Xyl-1 gene from A. cellulolyticus (FIG. 2). The primer pair used was Xyl For (5′-GTG GTG GAG CTC GCA ATT CGT TCA CGT TGA GG-3′) (SEQ ID NO: 14) and Xyl Rev (5′-GTG GTG TCT AGA ACC ATC GAG TGG GAG TGA CG-3′) (SEQ ID NO: 15). The underlined sequences indicate the added restriction sites for cloning, Sad and XbaI, respectively. PCR was performed using the following program using Pfu: an initial denaturation at 95° C. for 3 min, followed by 25 cycles of 95° C. for 1 min, 55° C. for 1 min, 70° C. for 1 min, and then a final extension at 70° C. for 5 min. The PCR yielded a 1.4-kb product, which is the xyl-1 gene (FIG. 3). Th...
example 2
Xyl-1 Expression in A. cellulolyticus
Materials and Methods
[0101]A. cellulolyticus cultures were grown in supplemented mineral medium as described previously (12). Oat spelt xylan, cellulose, cellobiose, or glucose were provided individually as carbon sources at 0.5%. Cells were grown to mid exponential phase or stationary phase and cells were harvested by centrifugation at 10,000×g for 15 min at 4° C. The cell pellet was frozen in liquid nitrogen and cells were disrupted by grinding with a mortar and pestle prechilled in liquid nitrogen, in the presence of sterilized sand. RNA was extracted using the RNeasy Plant Mini Kit (Qiagen), with several rounds of DNase digestion with RNase-free DNase (Qiagen), and cleaned with the RNeasy Plant Mini Kit. For the cultures grown on oat spelt xylan or cellulose, high molecular weight polyethylene glycol (at 1% w / v final concentration) was added to the cell lysate to reduce the amount of contaminating genomic DNA. It was found that several round...
example 3
Sequence Analysis of Xyl-1 Gene Promoter
[0106]The xyl-1 gene was determined to be on the negative strand and is separated by 147 by from the divergently oriented upstream gene, and 451 by from the downstream gene on the negative strand. The functions of both of these flanking genes appear to be unrelated to xylanases. Thus, xyl-1 does not appear to be part of a gene cluster or operon. Analysis of the 451-bp upstream promoter region revealed a putative Shine-Dalgarno ribosomal binding site (RBS) six nucleotides upstream of the translational start. The RBS sequence (5′ TGGAGG 3′) (SEQ ID NO: 20) is complementary to the conserved CCTCCT (SEQ ID NO: 21) sequence found at the 3′ end of the A. cellulolyticus 16S ribosomal rRNA, with only a one-base mismatch (FIG. 7). Putative-35 and -10 sequences were also identified (FIG. 7) that match well with the consensus promoter motifs proposed in the closely-related actinomycete, Streptomyces (20), and are likely to be the σ70 promoter sequences o...
PUM
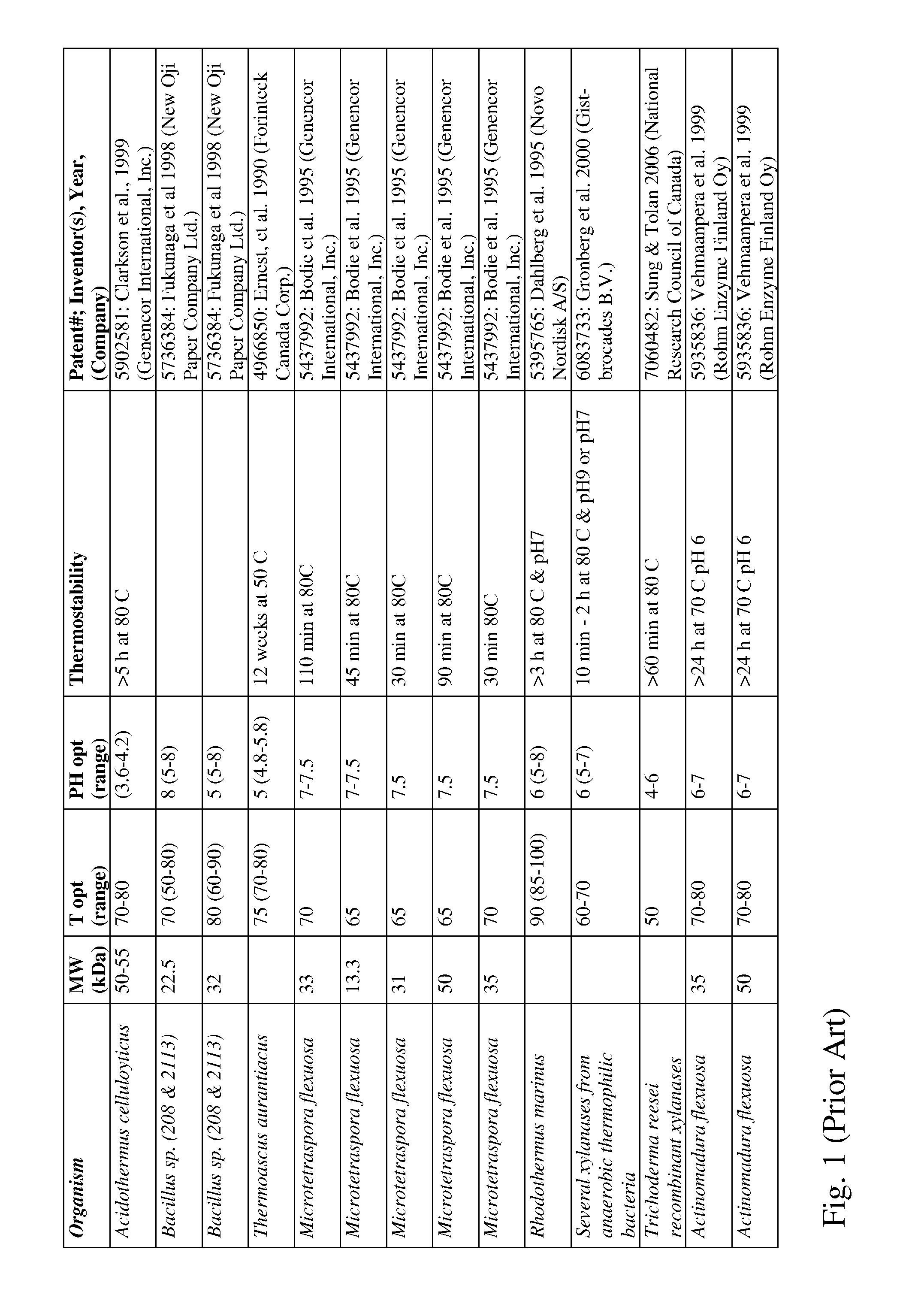
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com