Engineered osteochondral construct for treatment of articular cartilage defects
a technology of articular cartilage and constructs, which is applied in the field of treatment of articular cartilage defects, can solve the problems of less load-bearing tissue, complex autologous chondrocyte transplantation technique, and difficulty in restoring normal function, so as to achieve the effect of easy placement in the defect area and restoring normal function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0026]The present invention is susceptible of embodiment in various forms as will hereinafter be described with the understanding that the present disclosure is to be considered as an exemplification of the invention, and is not intended to limit the invention to the specific embodiments disclosed herein.
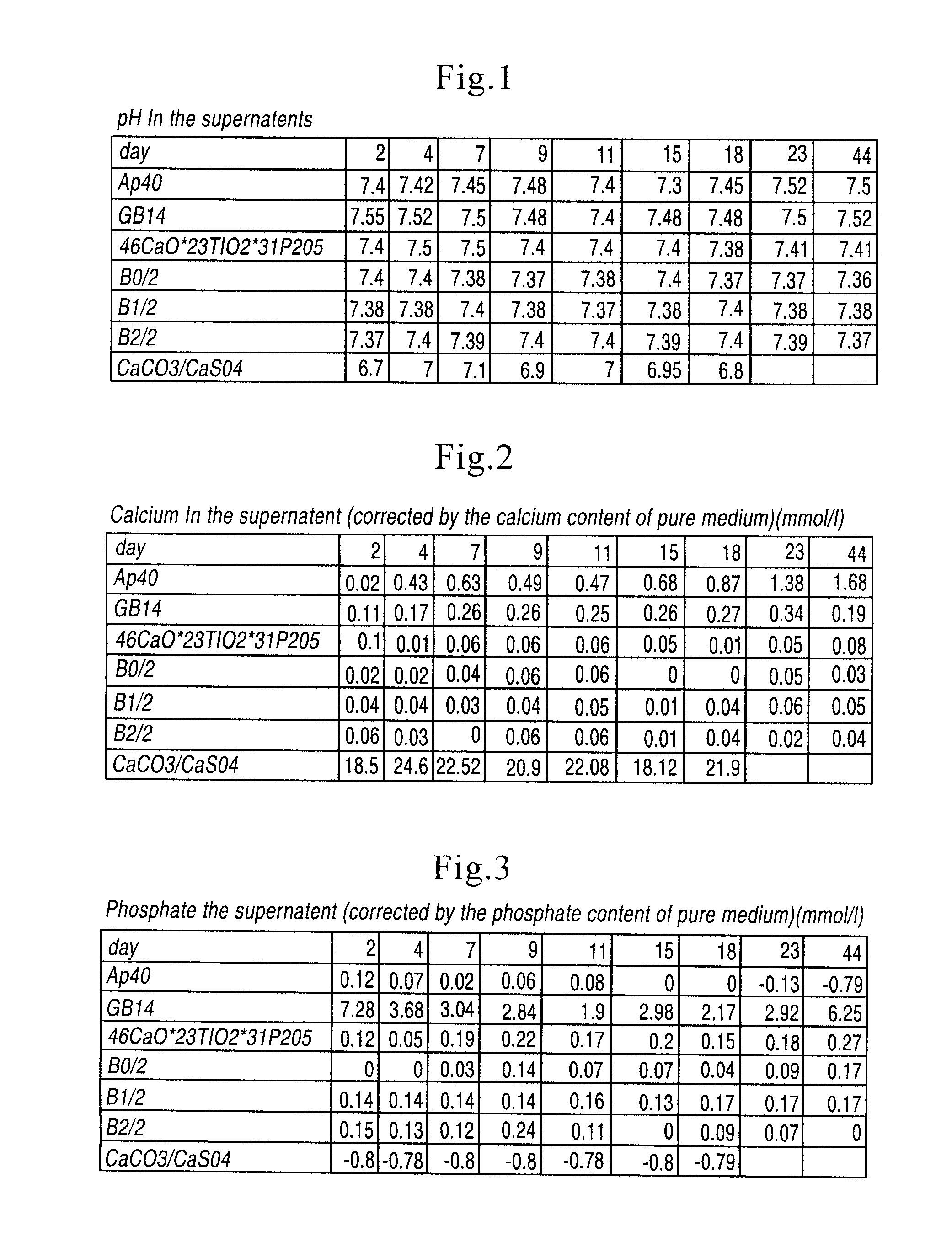
[0027]Sterile cancellous bone replacement structures were utilized for the in vitro grown cartilage replacements, which allow the fabrication of load-bearing constructs. Bone morphogenetic proteins (“BMP's”) from the cancellous bone plugs have a positive effect on chondrocyte differentiation in vitro by stimulating the formation of a native, chondrocyte-phenotype and proper matrix production by the cells. The highest stimulation effect of BMP's on chondrocytes can be observed, if BMP's are immobilized onto a carrier or retained in a biological matrix. In these carriers the natural BMP's of the bone are released by the demineralization but retained in the carrier matrix. For evaluati...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| depth | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com