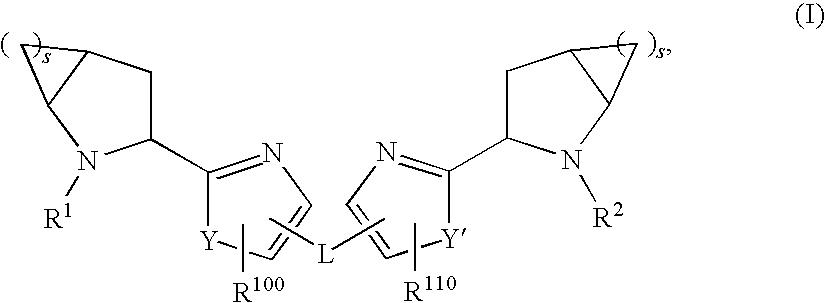
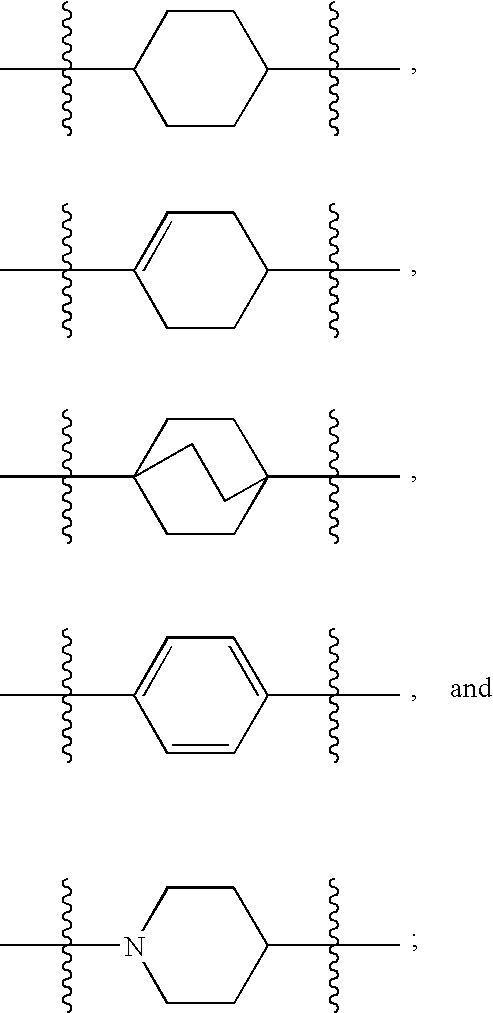
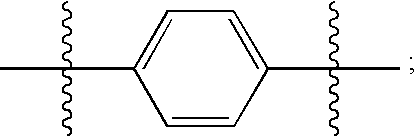
Hepatitis C Virus Inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0734]The present disclosure will now be described in connection with certain embodiments which are not intended to limit its scope. On the contrary, the present disclosure covers all alternatives, modifications, and equivalents as can be included within the scope of the claims. Thus, the following examples, which include specific embodiments, will illustrate one practice of the present disclosure, it being understood that the examples are for the purposes of illustration of certain embodiments and are presented to provide what is believed to be the most useful and readily understood description of its procedures and conceptual aspects.
[0735]Solution percentages express a weight to volume relationship, and solution ratios express a volume to volume relationship, unless stated otherwise. Nuclear magnetic resonance (NMR) spectra were recorded either on a Bruker 300, 400, or 500 MHz spectrometer; the chemical shifts (8) are reported in parts per million.
[0736]Purity assessment and low ...
example qc-1a
[0784]
[0785]To a solution of bicyclohexanone (5.05 g, 26.0 mmol) and 2,6-diterbutyl phenol (11.75 g, 57.2 mmol) in 200 mL of dry dichloromethane was added triflic anhydride (9.10 mL, 54.6 mmol). The resulting solution was stirred at room temperature overnight. The solvent was removed under vacuum. The residue was taken up in hexanes and filtered. The filtrate was washed with 1 N HCl and brine. The organic layer was dried with potassium carbonate and concentrated. The crude product was purified by a flash chromatography (silica gel, 5% ethyl acetate / hexanes) to give Example QC-1a as a white solid (8.22 g, 69.0%, mixture of diastereomers). 1H NMR (500 MHz, CDCl3) δ ppm 1.35-1.64 (m, 4H) 1.81-2.07 (m, 4H) 210-2.53 (m, 6H) 5.66-5.80 (m, 2H). LC / MS: Anal. Calcd. for [M+H]+ C14H17F6O6S2: 459.37; found (molecule did not ionize well in mass spec chamber).
example qc-1b
[0786]
[0787]To a flask with bis(pinacolato)diboron (2.79 g, 11.0 mmol), potassium phenolate (1.98 g, 15.0 mmol), PdCl2(PPh3)2 (0.21 g, 0.3 mmol) and triphenylphosphine (0.16 g, 0.6 mmol) was added 50 mL of dry toluene and (1a) (2.29 g, 5.0 mmol). The resulting mixture was stirred at 50° C. for 3 h. The reaction was quenched with water and extracted with toluene. The organic layers were combined, washed with brine, dried with MgSO4 and concentrated. The crude product was purified by flash chromatography (silica gel, 5-80% ethyl acetate / hexanes) to give Example QC-1b as a white solid (1.14 g, 55%, mixture of diastereomers). 1H NMR (500 MHz, CDCl3) δ ppm 1.10-1.19 (m, 2H) 1.24 (s, 24H) 1.31-1.43 (m, 2H) 1.73-1.90 (m, 4H) 1.97-2.29 (m, 6H) 6.52-6.58 (n, 2H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com