Natriuretic fusion proteins
a fusion protein and natriuretic technology, applied in the field of fusion proteins with diuretic, natriuretic, vasodilatory activity, can solve the problems of short half-life of proteins, limited previous therapeutic administration of peptides, and inability to achieve the effect of preventing bacterial infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Structural Modeling to Predict Minimum Linker Distance
[0198]Structural modeling was done to determine the linker requirements for the Fc and ANP fusions. With the both molecules oriented to minimize distance as well as minimize steric and electrostatic repulsions, a minimal distance from the C-terminus of ANP is 12 Å from the closest N-terminus of the Fc dimer and 17 Å from the other N-terminus of the Fc dimer. If the Fc dimer has only one ANP fused, a 4 to 6 aa minimum linker length would be suggested. With two ANP peptides bound to the Fc dimer, if only one ANP bound (in a 1:1 Fc dimer:NPRA ratio) then a minimum linker length of 9 aa for each linker would be suggested. For both ANP's to bind in a 1:2 Fc dimer:NPRA ratio, linkers with a minimum length of 12 aa would be suggested.
example 2
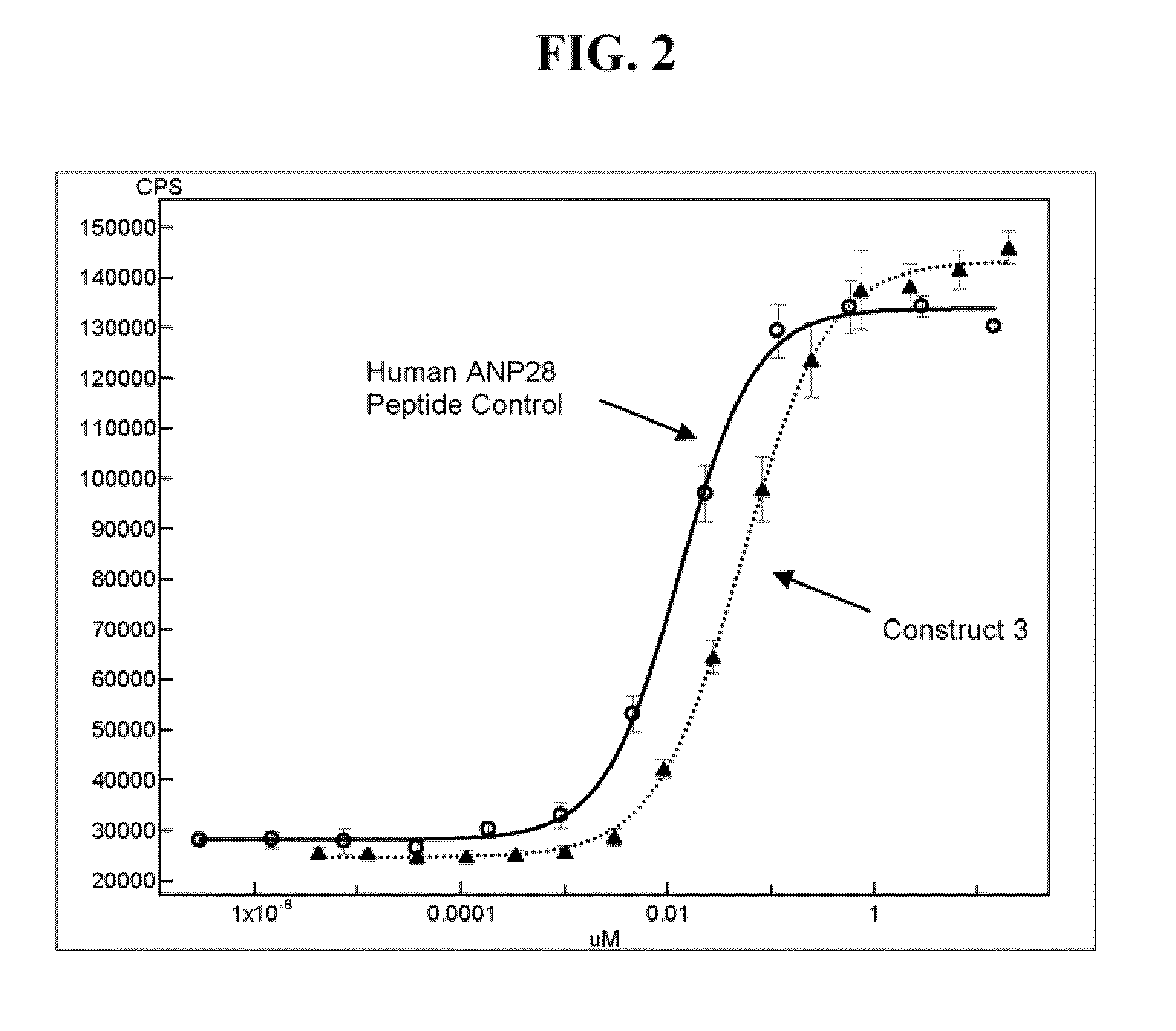
Synthesis and Characterization of Synthetic ANP Fusion Proteins
[0199]Synthetic chemistry was used to generate hANP28 peptides with linkers fused to human Fc of IgG1 isotype. The resulting semi-synthetic ANP-Fc fusion molecules generated are shown in Table 2. The linkers tested were glycine succinate (L1), GlyGly (L2), (GlyGlySer)3GlyGly (L4) (SEQ ID NO: 3) and Gly(SerGlyGly)2SerGly (L3) (SEQ ID NO: 2). The glycines (G) were used to add flexibility, while the polar serines (S) were built in to add solubility. The hANP28 peptides with linkers were linked to recombinantly produced Cys-Fc in two orientations: 1) C-terminus of hANP28+Linker fused to N-terminus of Cys-Fc [Orientation #1], and 2) N-terminus of Linker+hANP28 fused to N-terminus of Cys-Fc [Orientation #2]. The synthetic chemistry of the first orientation resulted in a complete peptide bonded structure while the chemistry of the second orientation left a succinate moiety in place of one amino acid of the fusion.
[0200]The semi...
example 3
Recombinant ANP Fusion Proteins
[0205]A production platform for the rapid generation of recombinant ANP-Fc fusion proteins was generated. The process starts with “base” Fc fusion vectors that allows DNA cassettes to be rapidly and seamlessly inserted onto the N-terminus of either IgG1 or IgG2 Fc. The Fc fusions to both IgG1 and IgG2 isotypes were generated in such a way that the hinge region is chopped down to the same CPPCP hinge residues thus ensuring that the linker extension and ANP fusion would be equally extended on the two isotypes. The respective DNA and protein sequences of the four recombinant ANP-Fc fusion proteins generated are represented by SEQ ID NOs: 24-31 (see, e.g., FIG. 1A-B). These fusion proteins each comprise a N-terminal mouse IgG kappa light chain signal sequence METDTLLLWVPGSTG (SEQ ID NO: 32) that is cleaved off and not part of the final protein product. The bolded The ANP-Fc fusion constructs were initially produced using 1 L transient mammalian expression ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Composition | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com