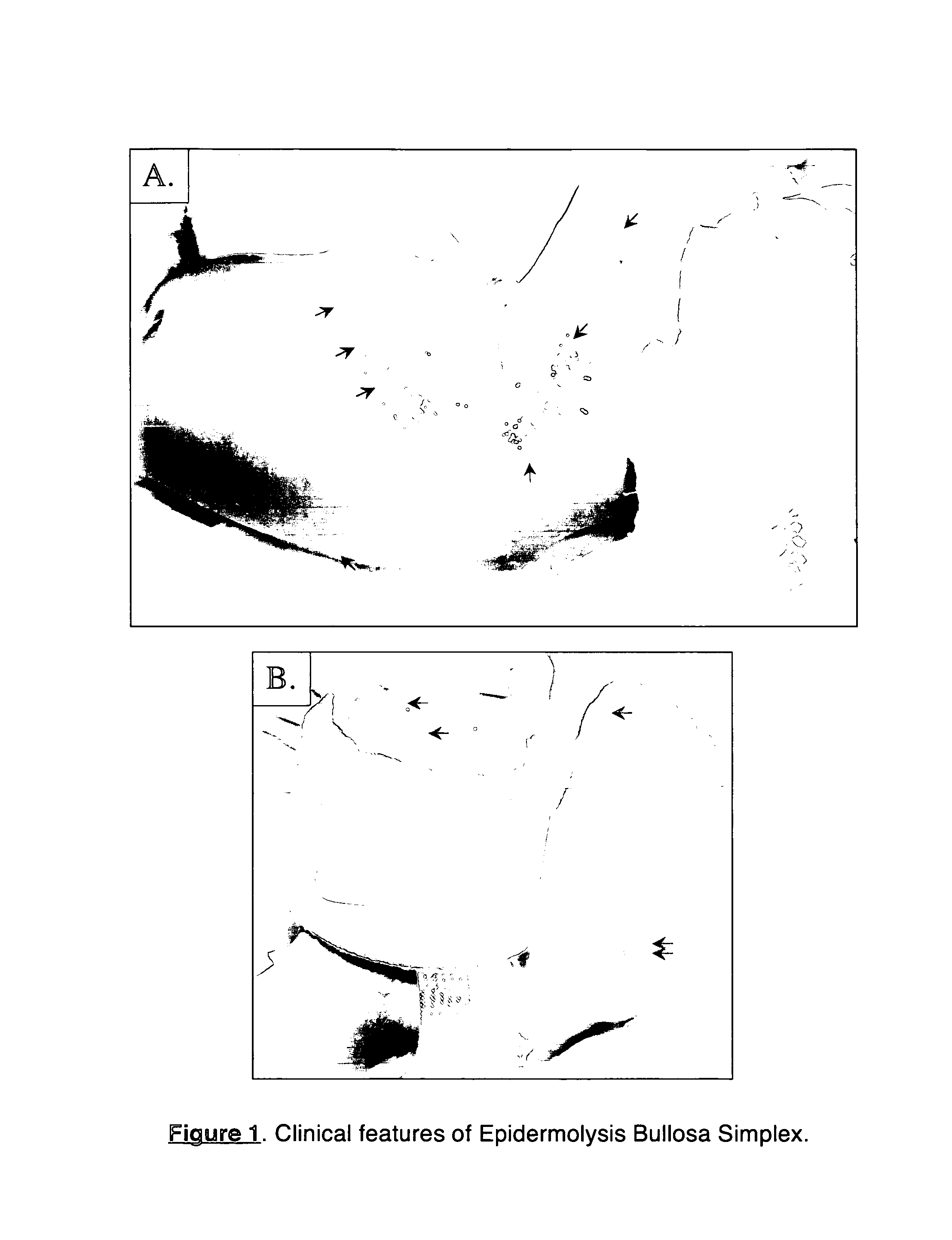
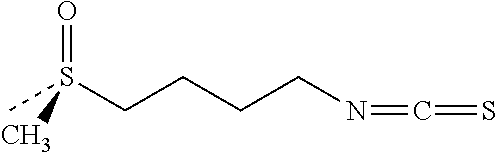
Use of nrf2 inducers to treat epidermolysis bullosa simplex and related diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
K14 Null and K5 Null Mouse Strains as Models for Very Severe EBS Disease
[0051]Introduction of null mutations at the K14 locus (Lloyd et al., 1995) and K5 locus (Peters et al., 2001) in mice essentially abrogate the keratin filament network in basal keratinocytes in the epidermis, and renders the keratinocytes acutely fragile in the face of physiological levels of mechanical trauma. The presence of small amounts of K15, a type I keratin related to K14, leaves a residual but wispy keratin filament network in basal keratinocytes of K14 null epidermis. Accordingly, K5 null mice show more extensive skin blistering and die sooner (before P0.5) than K14 null mice (P2-P3 in K14 null mice). Thus, these two mouse models represent very severe forms of the disease.
[0052]The K14 null mouse strain (Lloyd et al., 1995) has proven to be the more useful model for these studies. The selective fragility of epidermal basal cells and the associated trauma-induced skin blistering seen in K14-null mice mi...
example 2
Ectopic Expression of Gli2 Rescues Skin Blistering in K14 Null Mice but not in K5 Null Mice
[0053]Keratin 17 is a direct target for the transcription factor Gli, a powerful terminal effector of hedgehog signaling pathways (Bianchi et al., 2005). In Gli2TG transgenic mice (Grachtchouk et al., 2000), expression of the Gli2 coding sequence is controlled by the K5 gene promoter, thereby causing its accumulation in the basal layer of epidermis. Gli2TG mice appear normal at birth and in the days thereafter, but they develop epidermal hyperplasia as young adults, which progresses to basal cell carcinoma by 2-3 months of age. Availability of Gli2TG transgenic mice provided an opportunity to conduct a “proof of principle” experiment, whereby constitutive expression of Gli2TG transgene in the setting of K14− / − mice should cause a stable upregulation of K17 in basal keratinocytes of epidermis, and hence, rescue their oral and skin blistering. Conversely, the Gli2TG transgene should not rescue t...
example 3
Sulforaphane Selectively Induces K16 and K17 in Skin Keratinocytes in Vitro and in Vivo
[0055]To evaluate the effect of sulforaphane (SF) on keratin gene transcription in vitro, a mouse keratinocyte line (308 cells) was exposed to 1 μM SF in acetonitrile vehicle, and mRNA levels were measured at 12, 24, and 48 hours after treatment. Relative to vehicle treatment, SF-treated keratinocytes showed a significant increase in the mRNA levels of NQO1, a well-established SF target, at all time points as expected (Dinkova-Kostova, et al., 2006). Similarly to NQO1, K17 and K16 mRNAs were each elevated ˜2.5 fold at 12 h after SF treatment, but their induction was shorter-lived and levels returned to baseline by 24 hours. No significant change was measured for K5, K6a, K6b, K14 and K15 mRNA levels. Indirect immunofluorescence revealed an obvious induction of K17, but not K14, at the protein level.
[0056]At higher doses and in some specific contexts, SF induces apoptosis, or programmed cell death ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Phase | aaaaa | aaaaa |
| Resilience | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com