Electrosurgical systems and methods for removing and modifying tissue
a tissue removal and electrosurgical technology, applied in the field of electrosurgical systems, can solve the problems of undesirable collateral tissue damage in the region, rapid tissue heating, and the like of conventional electrosurgical devices and procedures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
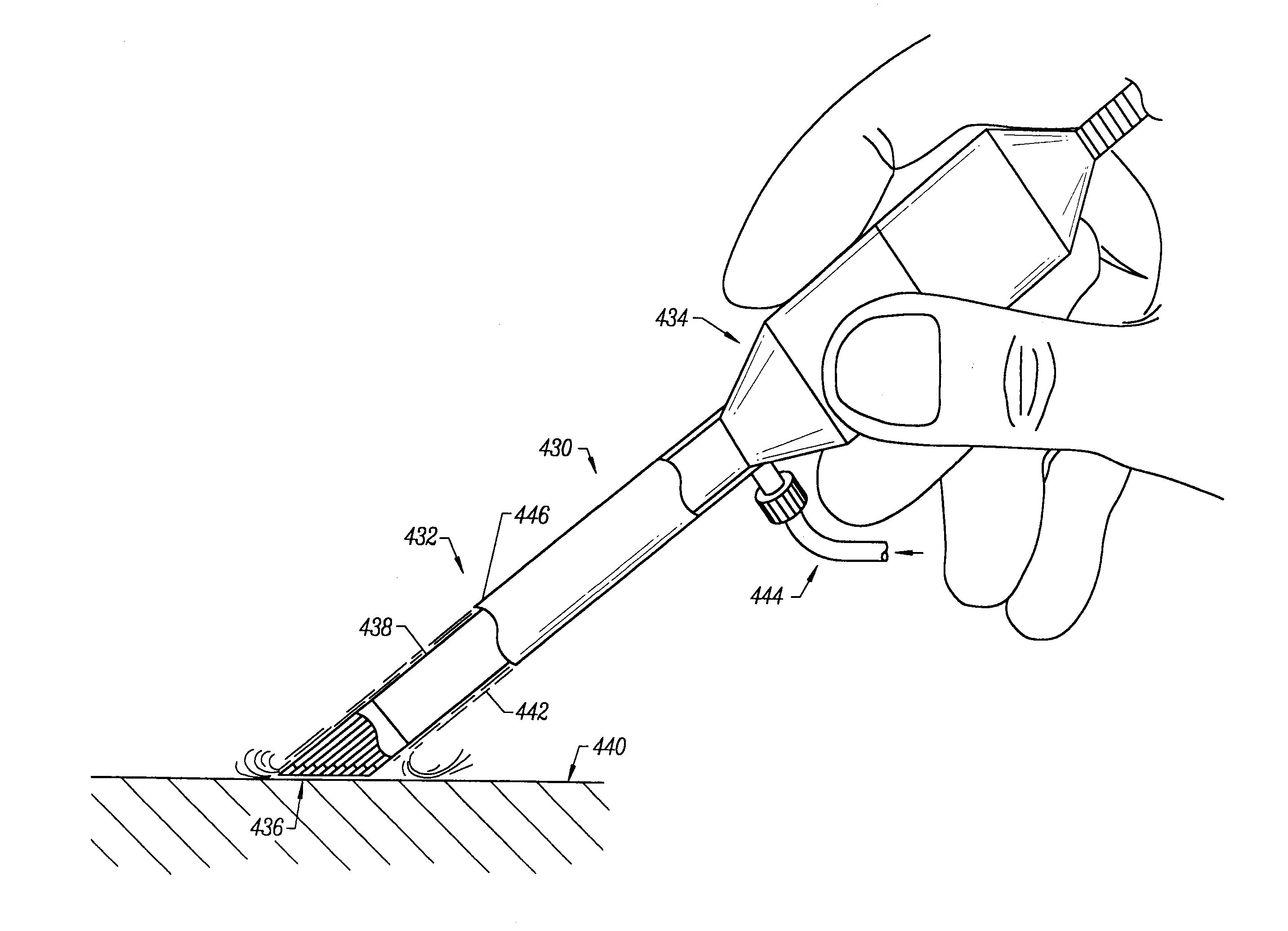
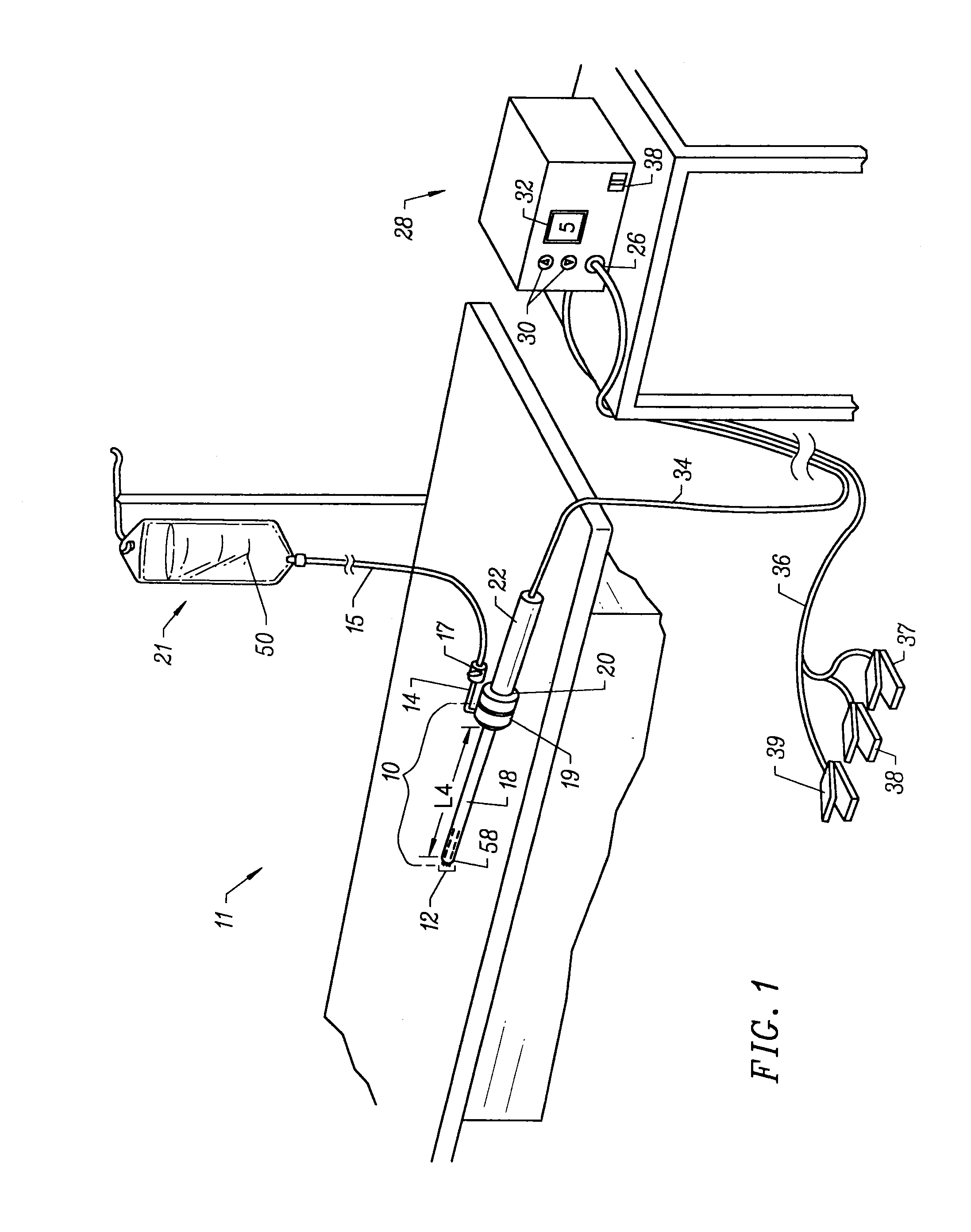
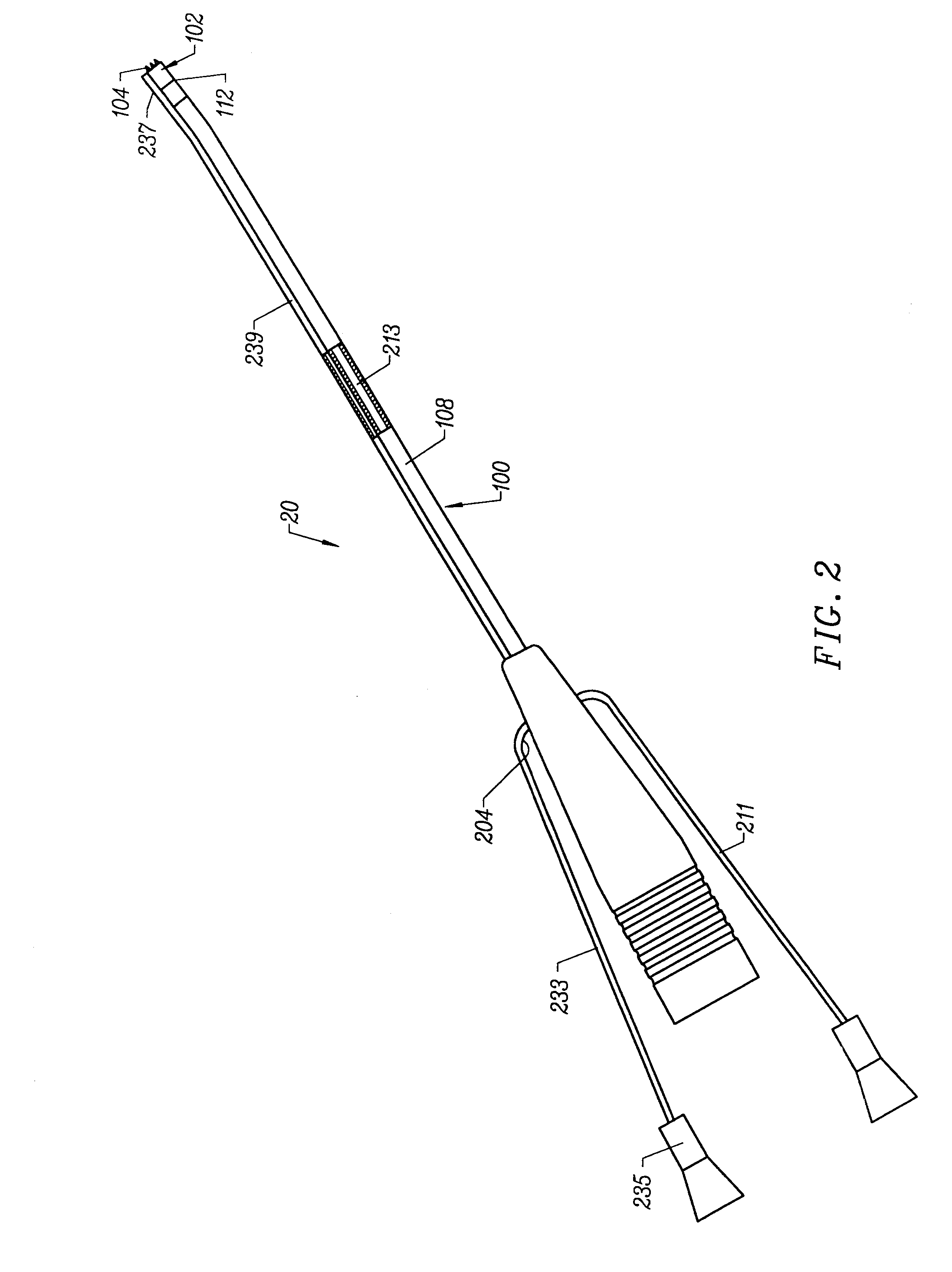
[0071]The present invention provides systems and methods for selectively applying electrical energy to a target location within or on a patient's body, particularly for cutting, ablating, and / or coagulating a tissue using a blade-like electrode. The instant invention also provides apparatus and methods for making incisions to access a tissue or organ within a patient's body, to dissect or harvest the tissue or organ from the patient, and to transect or otherwise modify the tissue or organ. In one aspect, the invention provides apparatus and methods for dissecting and harvesting blood vessels from a patient.
[0072]The present invention is useful in procedures where the target tissue or organ is, or can be, flooded or submerged with an electrically conductive fluid, such as isotonic saline. In addition, tissues which may be treated by the system and method of the present invention further include, but are not limited to, tissues of the heart, chest, knee, shoulder, ankle, hip, elbow, h...
PUM
| Property | Measurement | Unit |
|---|---|---|
| distance | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| energy level | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com