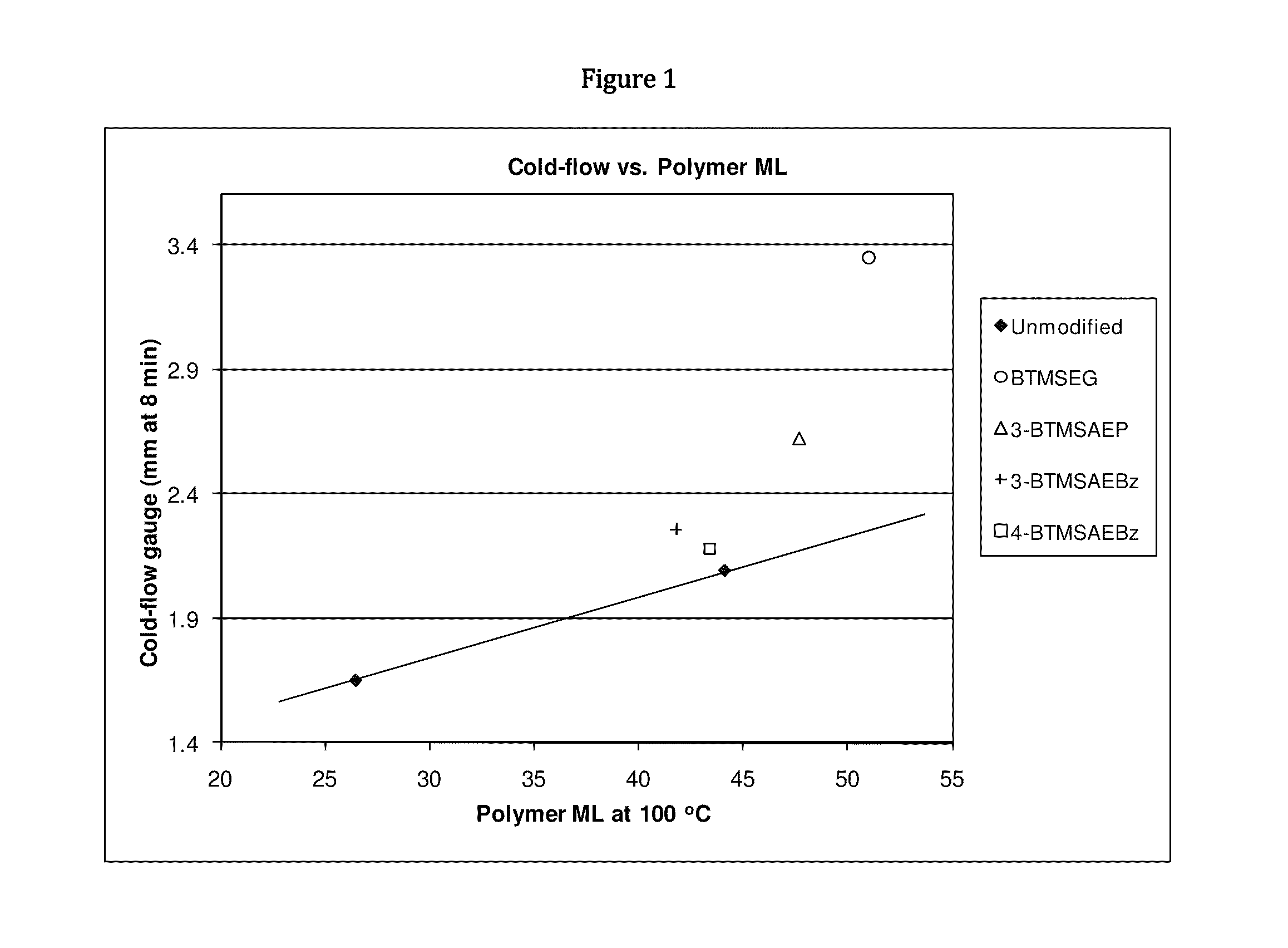
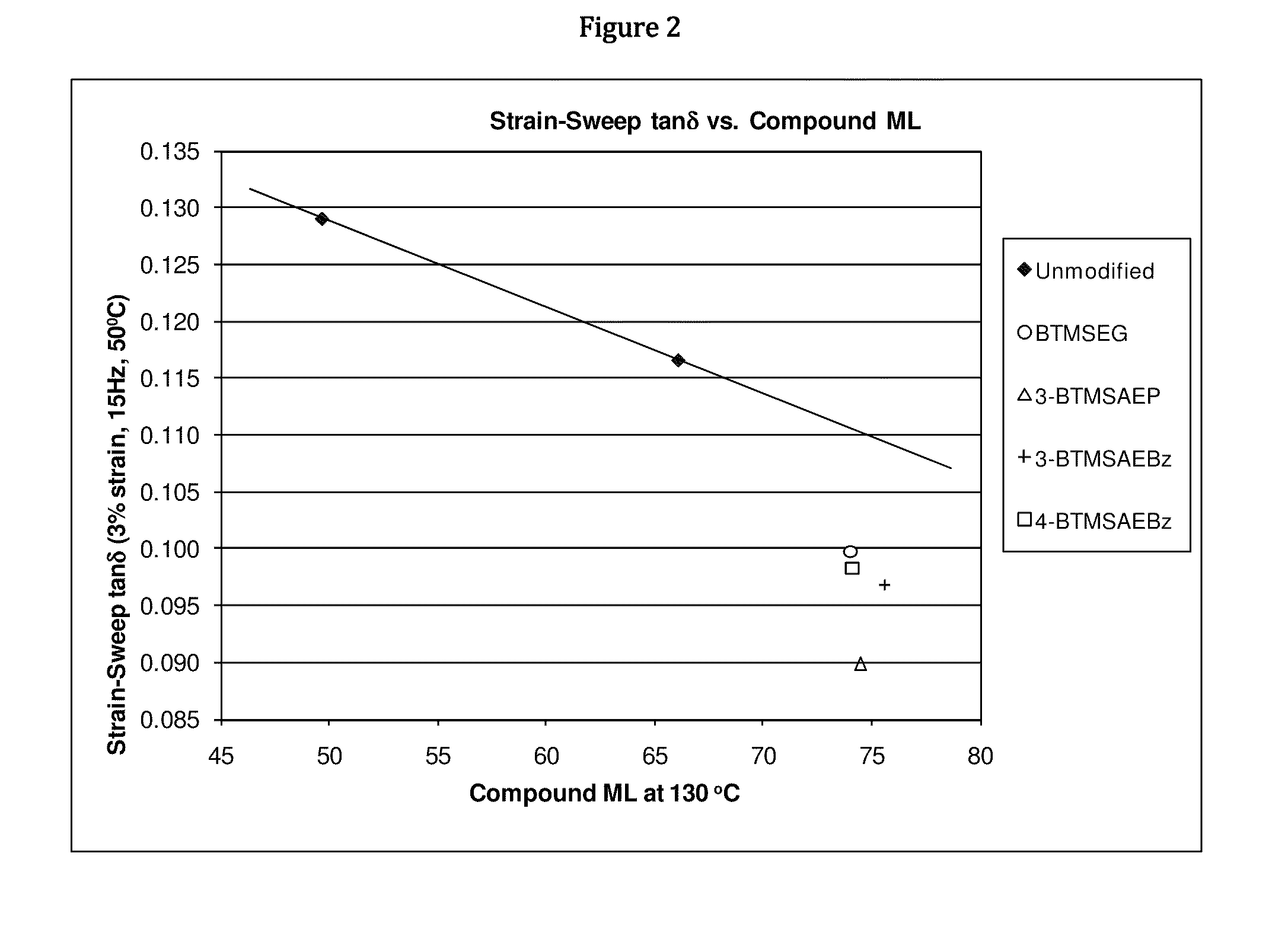
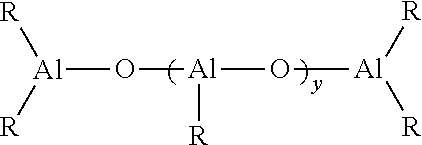
Functionalized polymers and methods for their manufacture
a technology of functionalized polymers and polymers, applied in the direction of transportation and packaging, tyre parts, special tyres, etc., can solve the problems of polymer high cold flow due to polymer hysteresis and unpredictable whether a reactive polymer can be functionalized by treatment with a particular functionalizing agen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Ethyl N,N-Bis(trimethylsilyl)glycinate (BTMSEG)
[0190]About 6.02 g of ethyl glycinate hydrochloride, 14.39 g of triethylamine, and 10 ml of toluene were mixed in a round-bottom reaction flask cooled with an ice bath. To this mixture was added, in a dropwise fashion, a solution of 21.1 g of trimethylsilyl trifluoromethanesulfonate in 50 ml of toluene. The resulting mixture was stirred at room temperature for 19 hours to give a biphasic mixture. The top layer was transferred to another flask, and the bottom layer was extracted with 40 ml of toluene. The combined toluene solution was evaporated under vacuum. The residue was extracted with 100 ml of hexane, and the hexane layer was evaporated under vacuum, yielding ethyl [bis(trimethylsilyl)amino]acetate, also known as ethyl N,N-bis(trimethylsilyl)glycinate (BTMSEG), as a colorless liquid (9.23 g, 87% yield). The 1H NMR data (C6D6, 25° C., referenced to tetramethylsilane) of the product are listed as follows: δ 3.89 (quartet...
example 2
Synthesis of Ethyl 3-[Bis(trimethylsilyl)amino]propionate (3-BTMSAEP)
[0191]About 7.23 g of ethyl 3-aminopropionate hydrochloride, 15.71 g of triethylamine, and 10 ml of toluene were mixed in a round-bottom reaction flask cooled with an ice bath. To this mixture was added, in a dropwise fashion, a solution of 23.00 g of trimethylsilyl trifluoromethanesulfonate in 50 ml of toluene. The resulting mixture was stirred at room temperature for 39 hours to give a biphasic mixture. The top layer was transferred to another flask, and the bottom layer was extracted with 40 ml of toluene. The combined toluene solution was evaporated under vacuum. The residue was extracted with 100 ml of hexane, and the hexane layer was evaporated under vacuum, yielding ethyl 3-[bis(trimethylsilyl)amino]propionate (3-BTMSAEP) as a colorless oil (11.03 g, 90% yield). The 1H NMR data (C6D6, 25° C., referenced to tetramethylsilane) of the product are listed as follows: δ 3.91 (quartet, 2H, OCH2 protons), 3.21 (mult...
example 3
Synthesis of Ethyl 3-[Bis(trimethylsilyl)amino]benzoate (3-BTMSAEBz)
[0192]About 8.37 g of ethyl 3-aminobenzoate, 11.80 g of triethylamine, and 10 ml of toluene were mixed in a round-bottom reaction flask cooled with an ice bath. To this mixture was added, in a dropwise fashion, a solution of 25.91 g of trimethylsilyl trifluoromethanesulfonate in 50 ml of toluene. The resulting mixture was heated to reflux for 30 hours to give a biphasic mixture. The top layer was transferred to another flask, and the bottom layer was extracted with 40 ml of toluene. The combined toluene solution was evaporated under vacuum. The residue was extracted with 100 ml of hexane, and the hexane layer was evaporated under vacuum, yielding ethyl 3-[bis(trimethylsilyl)amino]benzoate (3-BTMSAEBz) as a yellow oil (14.50 g, 92% yield). The 1H NMR data (C6D6, 25° C., referenced to tetramethylsilane) of the product are listed as follows: δ 7.95 (multiplet, 1H, aromatic proton), 7.91 (multiplet, 1H, aromatic proton)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com