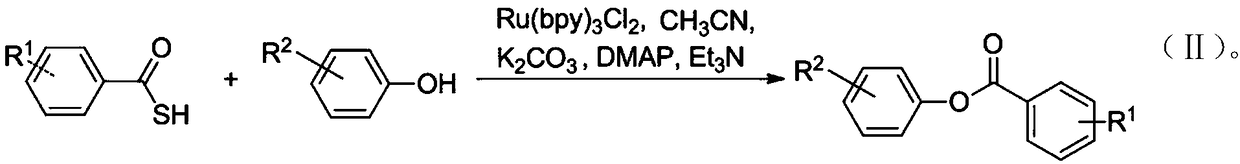
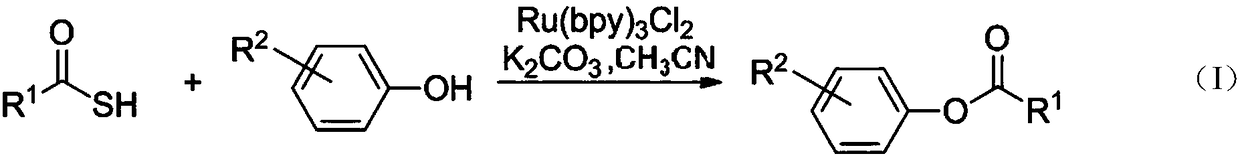Preparation method for synthesis of phenolic ester through thiocarboxylic acid mediated visible light catalyzed phenol acylation reaction
A technology of thiocarboxylic acid and catalyzed phenolic acyl, which is applied in the field of preparation of phenolic ester by catalyzed phenolic acylation reaction under visible light, achieving the effects of mild reaction conditions, strong applicability, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Add 1.0 mmol of aromatic thiocarboxylic acid substrates (such as Dissolve in 5mL of acetonitrile, add 0.01mmol of terpyridine hexahydrate ruthenium dichloride and 0.1mmol of potassium carbonate to the reaction system, stir for three hours in an open environment at room temperature under the irradiation of a 45W fluorescent lamp, then add 0.5mmol of phenolic base things (such as 1.0mmol triethylamine and 0.1mmol N, N-lutidine, after stirring for 10 minutes, pour the reaction mixture into 10mL water, extract four times with 10mL ethyl acetate, combine the organic phases and wash with 15mL saturated brine, After the organic phase was dried over anhydrous sodium sulfate, the organic solvent was removed by rotary evaporation, and the crude product was purified through a section of silica gel chromatography (n-hexane:ethyl acetate=20:1-5:1) about 10 cm long to obtain the corresponding Phenol ester compound 3, compound 5, compound 7 and compound 12.
[0019] Spectral data ...
Embodiment 2
[0080] 1.0mmol aliphatic thiocarboxylic acid substrate (such as and 0.5mmol phenolic substrate (such as Dissolve in 5mL of acetonitrile, add 0.01mmol hexahydrate terpyridyl ruthenium dichloride and 1.0mmol potassium carbonate to the reaction system, stir for 3-6 hours under sunlight in an open environment at room temperature, then pour the reaction mixture into Extract four times with 10 mL of ethyl acetate in 10 mL of water, combine the organic phases and wash with 15 mL of saturated brine, dry the organic phase with anhydrous sodium sulfate, remove the organic solvent by rotary evaporation, and pass the crude product through a silica gel chromatography column about 10 cm long (n-hexane:ethyl acetate=20:1) for purification, the corresponding phenolic ester compound 9 and compound 11 will be obtained.
[0081] Spectral data are as follows:
[0082]
[0083] 4-bromophenyl acetate 9a
[0084] Compound 9a was obtained with a yield of 56%, a total of 60.2 mg, as a colorles...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com