Electrochemical oxidation synthesis method for amide and application thereof
A technology of oxidative synthesis and synthesis method, applied in electrolysis process, electrolysis components, electrolysis organic production and other directions, can solve the problems of toxic activation reagents, expensive catalysts, etc., and achieve the effects of less by-products, high yield, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
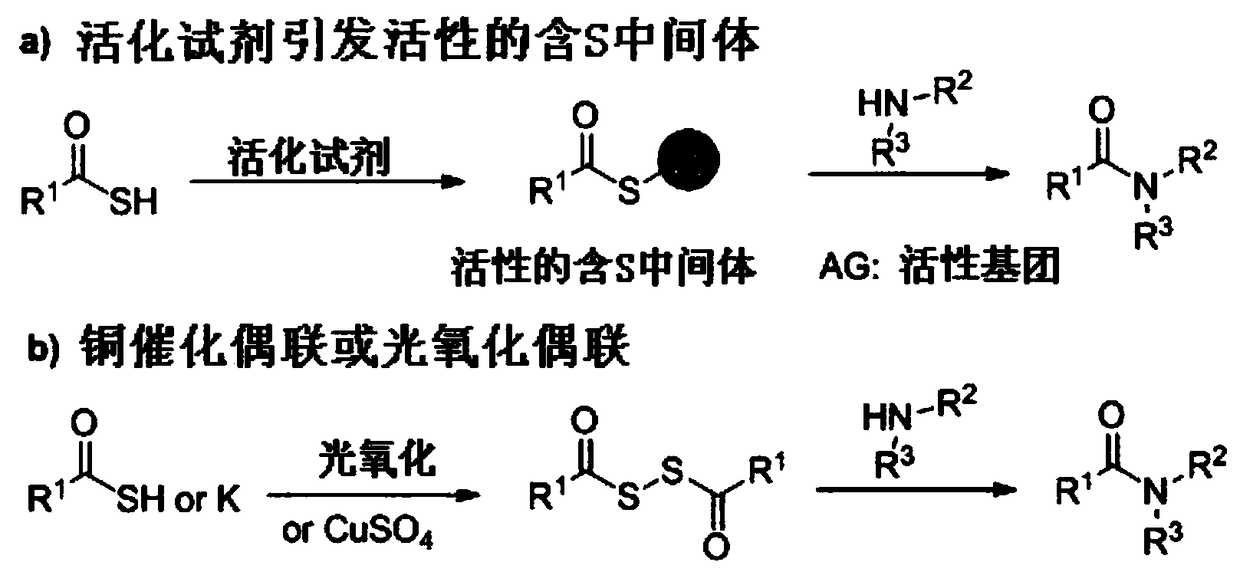
Method used
Image
Examples
Embodiment 1
[0031] The effect of different dielectrics on the electrochemical oxidation of amides:
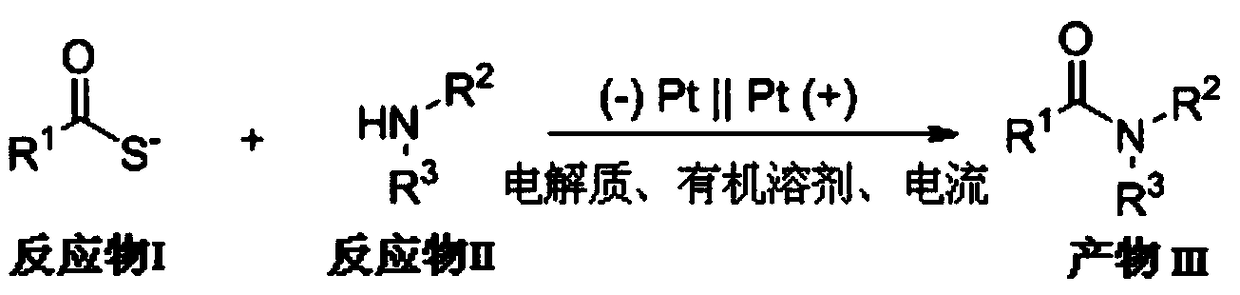
[0032] The general reaction formula is as figure 1 Shown:
[0033] (1) Electrochemical oxidation: First, add 1mmol of potassium thioacetate and 0.5mmol of aniline into the reaction test tube, add 0.50mmol of tetrabutylammonium tetrafluoroborate as the electrolyte, and 5.0mL of acetonitrile as the solvent and stirring bar. Install two 1.0cm×1.0cm platinum electrodes in the test tube as the cathode and anode respectively, and react for 24 hours after passing a current of 1.0mA;
[0034] (2) washing and drying: the mixture after the reaction is filtered, and the filtrate is successively washed with saturated NaHCO 3 Solution, saturated brine and pure water were washed and extracted, and the organic phase after washing and extraction was dried with anhydrous sodium sulfate and then rotary evaporated to obtain a crude product;
[0035] (3) Purification of amide compound: the crude product i...
Embodiment 2
[0039] Effects of different organic solvents on the synthesis of amides by electrochemical oxidation:
[0040] (1) Electrochemical oxidation: first, add 1 mmol of potassium thioacetate and 0.5 mmol of aniline into the reaction test tube, add 0.50 mmol of tetrabutylammonium tetrafluoroborate as electrolyte, and 5.0 mL of dichloromethane as solvent and stirring bar, Install two 1.0cm×1.0cm platinum electrodes in the reaction test tube as the cathode and anode respectively, and react for 24 hours after passing a current of 1.0mA;
[0041] (2) washing and drying: the mixture after the reaction is filtered, and the filtrate is successively washed with saturated NaHCO 3 Solution, saturated brine and pure water were washed and extracted, and the organic phase after washing and extraction was dried with anhydrous sodium sulfate and then rotary evaporated to obtain a crude product;
[0042] (3) Purification of amide compound: the crude product was purified by column chromatography, an...
Embodiment 3
[0045] (1) Electrochemical oxidation: first, add 1mmol of potassium thioacetate and 0.5mmol of aniline into the reaction test tube, add 0.50mmol of tetrabutylammonium tetrafluoroborate as electrolyte, 5.0mL of ethyl acetate as solvent and stirring bar, Install two 1.0cm×1.0cm platinum electrodes in the reaction test tube as cathode and anode respectively, and react for 12h after passing a current of 12.0mA;
[0046] (2) washing and drying: the mixture after the reaction is filtered, and the filtrate is successively washed with saturated NaHCO 3 Solution, saturated brine and pure water were washed and extracted, and the organic phase after washing and extraction was dried with anhydrous sodium sulfate and then rotary evaporated to obtain a crude product;
[0047] (3) Purification of amide compound: the crude product was purified by column chromatography, and the eluent was a mixed solvent of petroleum ether and ethyl acetate with a volume ratio of 2:1 to obtain the amide produc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com