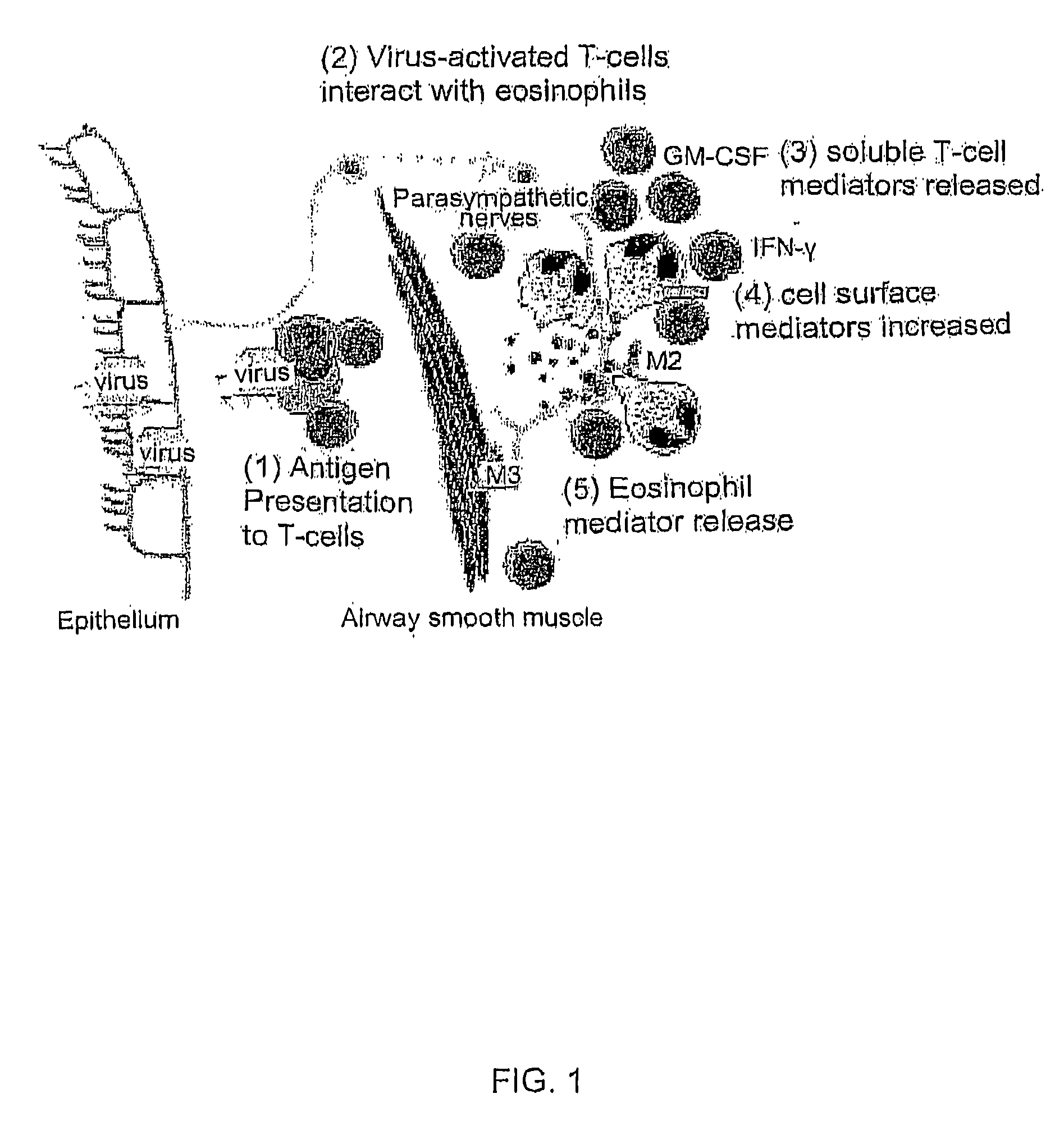
Activation of innate and adaptive immune responses by a ginseng extract
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Effect of CVT-E002 on Virus Replication In Vitro
[0097]The ability of various doses of CVT-E002 to inhibit virus replication in murine monocytic cells in vitro was investigated. Various doses (0, 10, 100 and 500 μg / ml) of CVT-E002 and HT-1001 (an exemplary ginseng fraction comprising ginsenosides Rb1 and Rg1 and described in U.S. Pat. No. 6,083,932) as control (250-2000 μg) were dissolved in PBS buffer and diluted to final concentrations in complete tissue culture medium and added to RAW-264 murine macrophage cells at 37° C. in vitro. Following treatment of the cells for 24 or 48 hours, treated and untreated cell cultures were exposed to herpes simplex virus type 2 (HSV-2) or vesicular stomatitis virus (VSV). Virus replication was assessed using plaque assays. Supernatants of CVT-E002 and control cultures were collected at various times following treatment and assessed for type I IFNs, TNFα, IL-6 and nitric oxide (NO) using ELISA. Significantly elevated levels of IL-6 (FIG. 8A), IFNβ...
example 2
Mechanism by which Anti-Viral Activity is Induced by CVT-E002
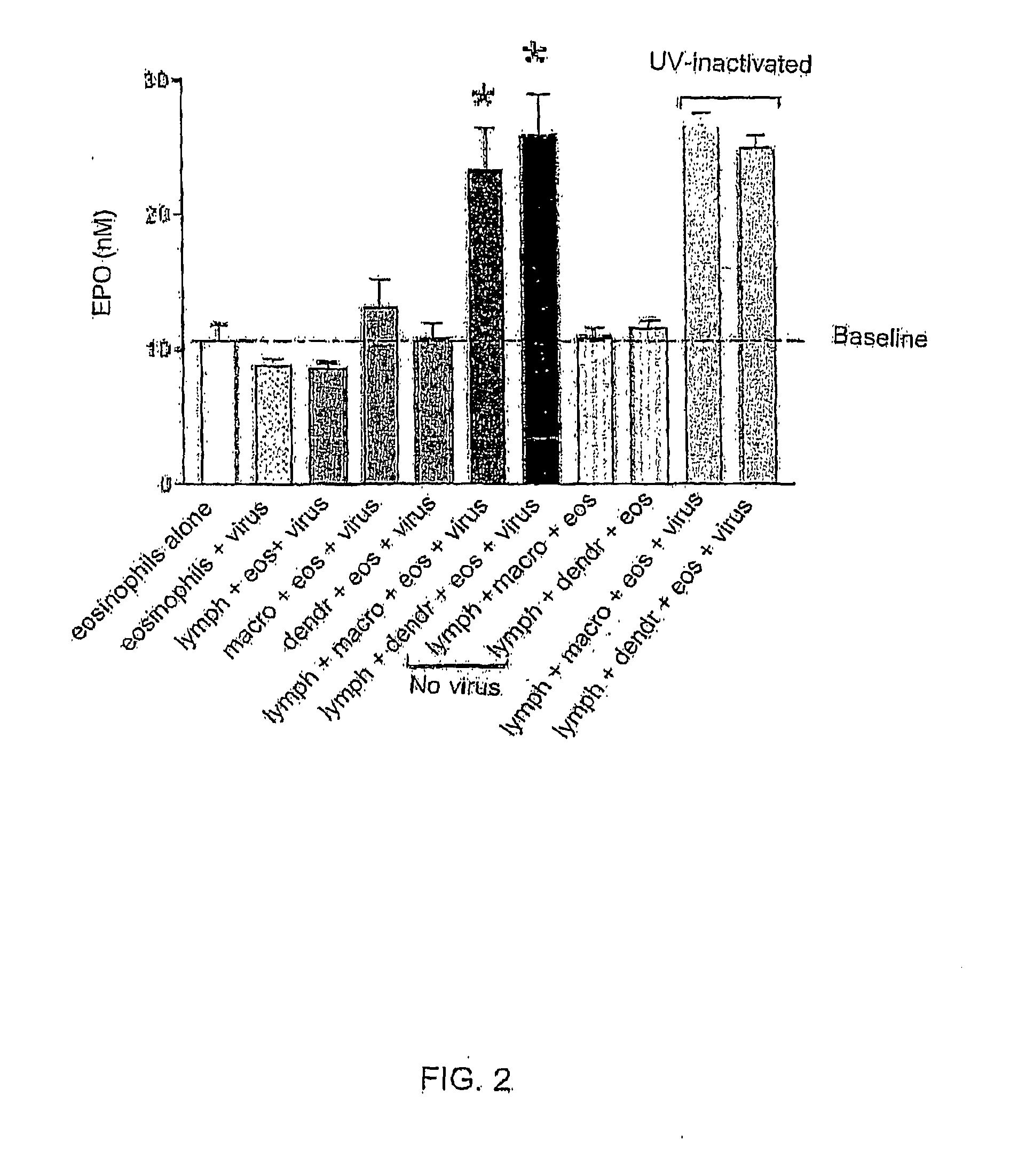
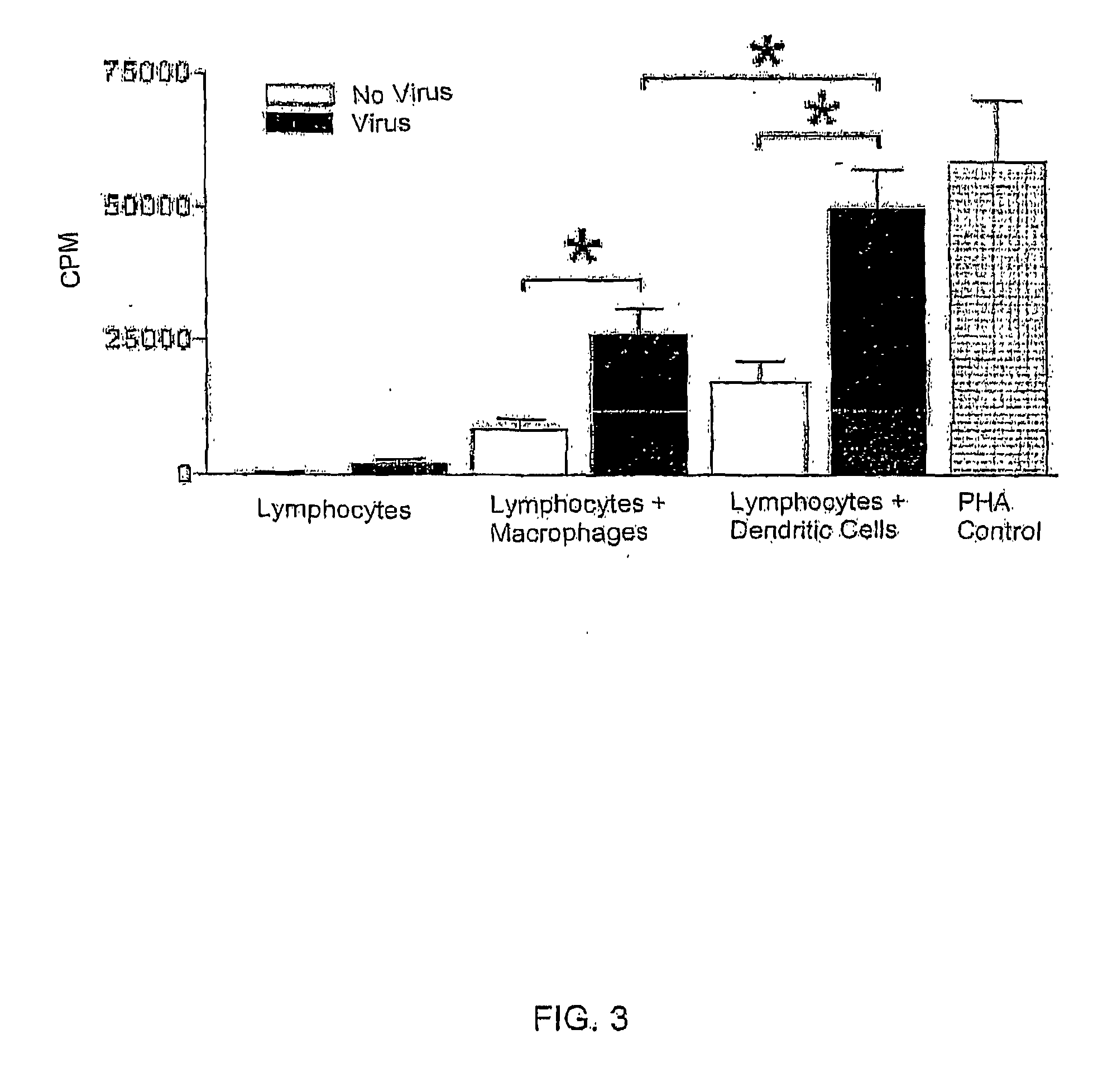
[0099]Dendritic cells (DCs) were co-cultured with lymphocytes and flowcytometry was used to characterize the lymphocytes. LPS stimulation was used as a positive control. RSV and PIV were found to induce proliferation of CD3+CD4+CD25+ T cells in the presence of dendritic cells (DCs) (FIG. 4, n=3 each). Compared to uninfected DCs cultured with lymphocytes, RSV induces production of interferons α and γ, TNF α, RANTES, IL-1, 2, 4, 10, 12, 13, and 15 (FIG. 5, n=1). The data are represented in relation to respective uninfected controls (given a value of 1). CVT-E002 with T cells alone induced release of IFNγ and IL12. CVT-E002 with DCs alone induced release of TNF, IFNγ, IL-1, 10, 12, and 15. Adding CVT-E002 to the cell culture with virus greatly augmented the cytokine / chemokine release. In addition, compared to uninfected cells, CVT-E002 could induce lymphocyte proliferation in the presence of DCs without virus (FIG. 6).
example 3
Effect of CVT-E002 on Dendritic Cell Function
[0100]Dendritic cells (DCs) were derived from blood monocytes by treatment with GM-CSF and IL-4 for seven days. DCs were incubated in the presence or absence of 5 mg / ml self-quenched DQ-Ovalbumin (DQ-OVA) and CVT-E002. DQ-OVA is a fluorescent dye attached to ovalbumin. DQ-OVA's fluorescence is quenched as an intact molecule, but when degraded through antigen presentation by DCs, the fluorescent dye is released and emission is allowed.
[0101]FIG. 7A shows the subpopulations of cells. “DC-like” cells are consistent by size and granularity with mature dendritic cells while “Not DC” are immature DC as well as differentiation-refractant monocytes and T-cells. LPS-treated cells were used as a positive control for DC maturation. Using flow cytometry to determine size and granularity, there appeared to be a subpopulation of monocytes, which are believed to represent an immature phenotype (Not-DC) as compared to the mature DC (DC-like). This impres...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap