Chromatography of polyolefin polymers
a technology of polyolefin polymer and chromatography, which is applied in the field of liquid chromatography, can solve the problems of limited separation efficiency obtained by prior art methods, tref and crystaf cannot be used to analyze amorphous polyolefin polymers, and tref and crystaf require a relatively long analysis tim
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
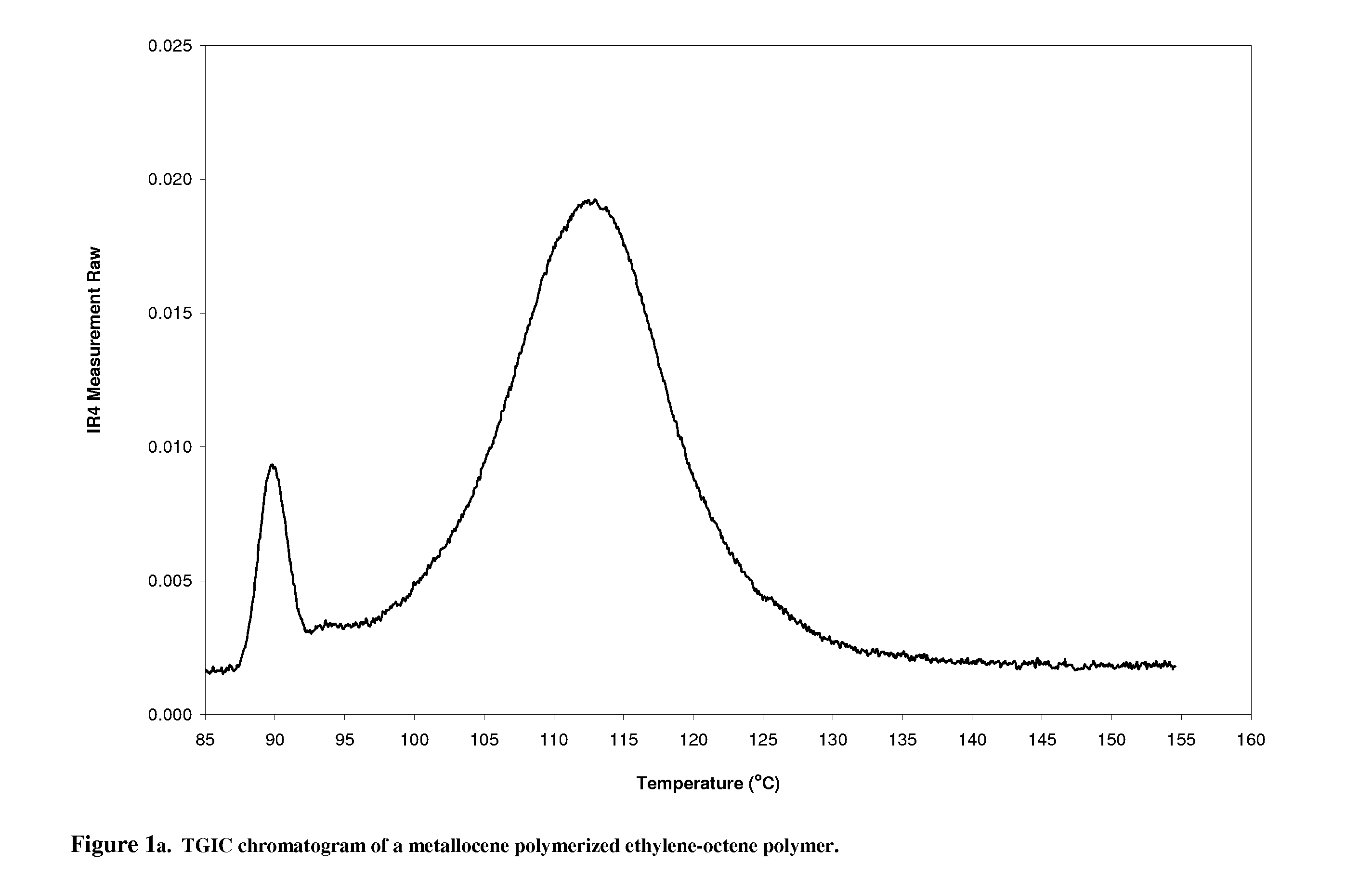
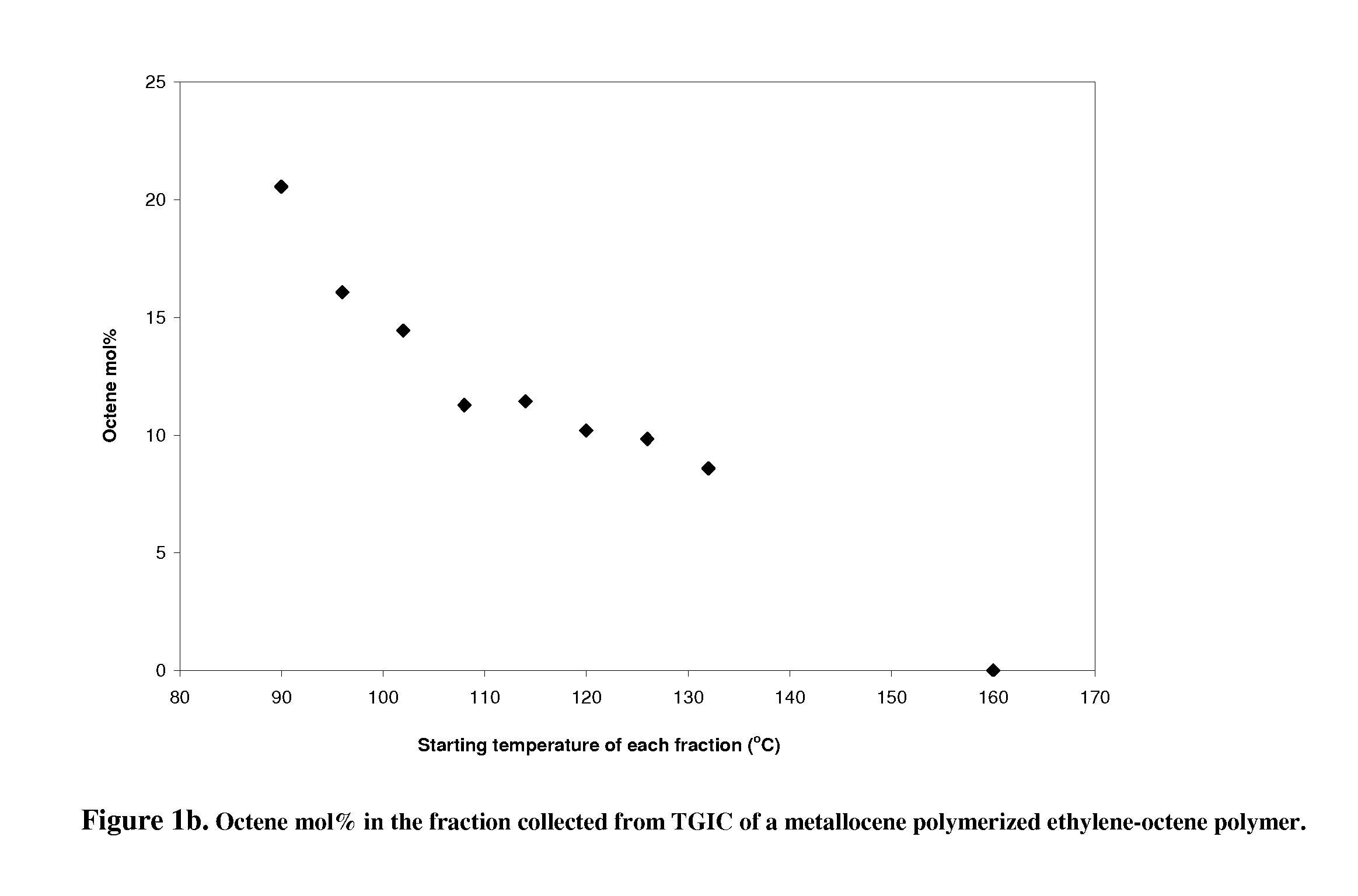
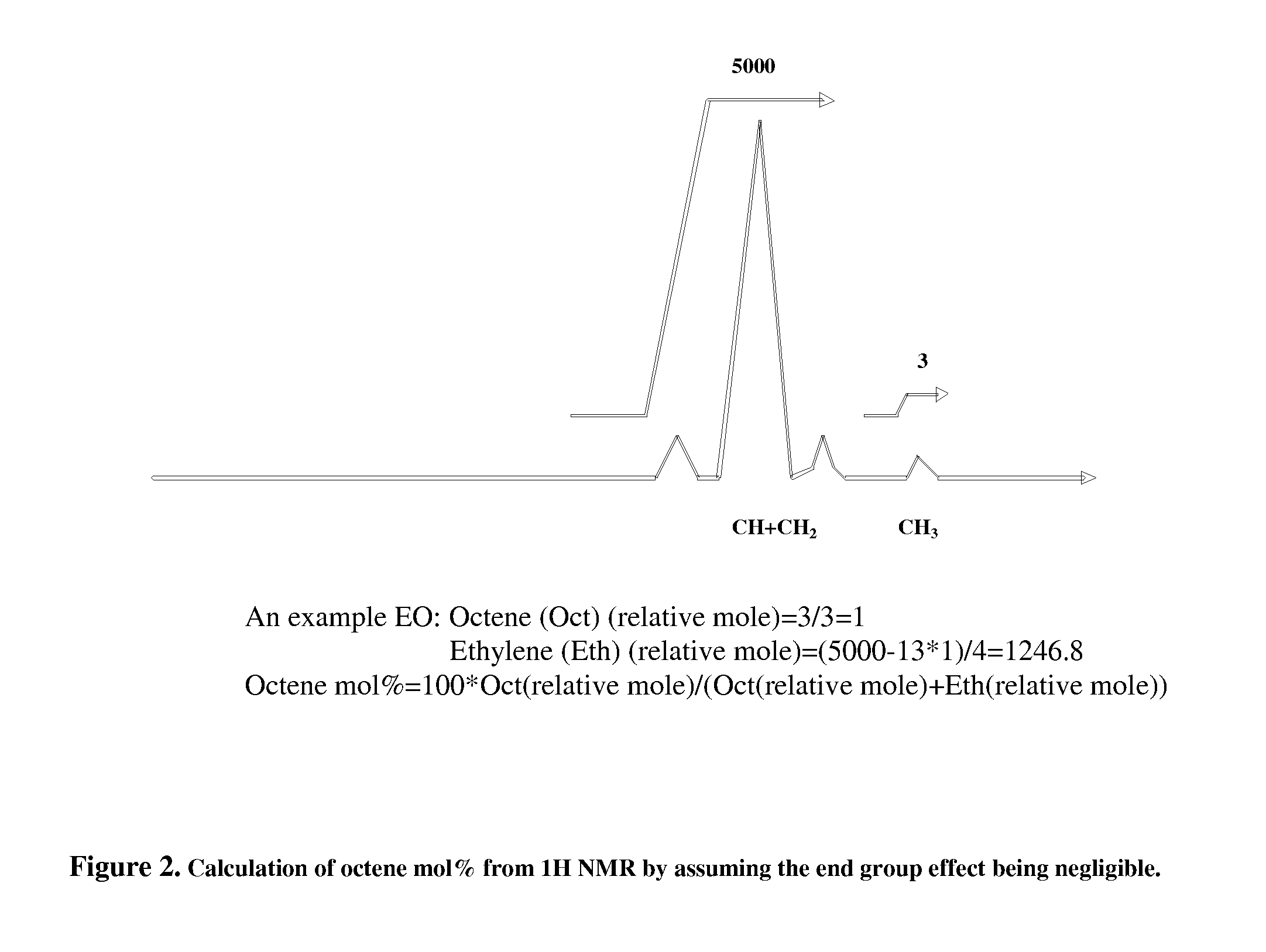
[0023]of the thermal gradient interaction chromatography. A metallocene polymerized ethylene-octene copolymer product (EO-1) is chosen for fractionation. EO-1 has a melt index of 0.82 g / 10 minutes and a density of 0.885 g / cm3; the sample for use in TGIC is prepared by weighing approximately 32 mg of polymer into a 10 ml GC glass vial, which is capped and placed in Crystallization Elution Fractionation (CEF) (PolymerChar, Spain) auto sampler. The instrument adds o-dichlorobenzene (ODCB), containing 300 ppm butylated hydroxytoluene (BHT) as an oxidation inhibitor, to the vial, producing a solution that is approximately 4 mg / mL in polymer. The dissolution is done by the autosampler at 160° C. for 90 minutes. The CEF is equipped with an IR-4 detector operating at 150° C. The delay volume (the volume that the first polymer fraction has to travel before reaching the detector) is 1.5 ml.
[0024]The column is a 10 cm long HYPERCARB column, part number 35005-104646, and the mobile phase is ODC...
example 2
[0029]the thermal gradient interaction chromatography on polymerized ethylene-octene (EO) polymers and blends. EO-2 has a melt index of 1 g / 10 minutes and a density of 0.865 g / cm3. EO-3 has a melt index of 66 g / 10 minutes and a density of 0.882 g / cm3. Blend #1 is 50:50(wt / wt) solution blend of a high density homopolymer polyethylene at melt index of 1 g / 10 min and density of 0.953 g / cm3, and EO-3. Blend #2 is a 50:50(wt / wt) solution blend of isotactic polypropylene at MFR (ASTM D 1238 condition 2.16 kg / 230° C.) of 13 g / 10 minutes and NIST SRM linear polyethylene 1484a.
[0030]The dissolution time is 120 minutes. The polymer solutions are loaded onto the column at 100° C. The polymer solution is kept at 100° C. for 2 minutes, and then cooled down to 80° C. at 20° C. / min, and kept at 80° C. for 5 minutes for thermal equilibrium, Polymer solution is eluted from 80° C. to 165° C. at 4° C. / min at a flow rate of 0.5 ml / min. Other experimental conditions are the same as Example 1. The chroma...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Retention factor | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com