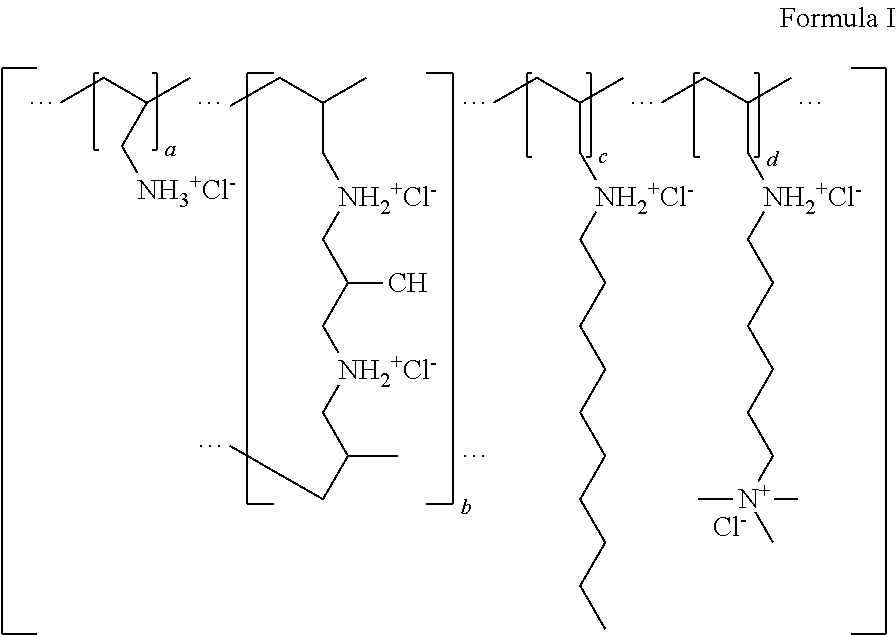
Crosslinked Polymers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
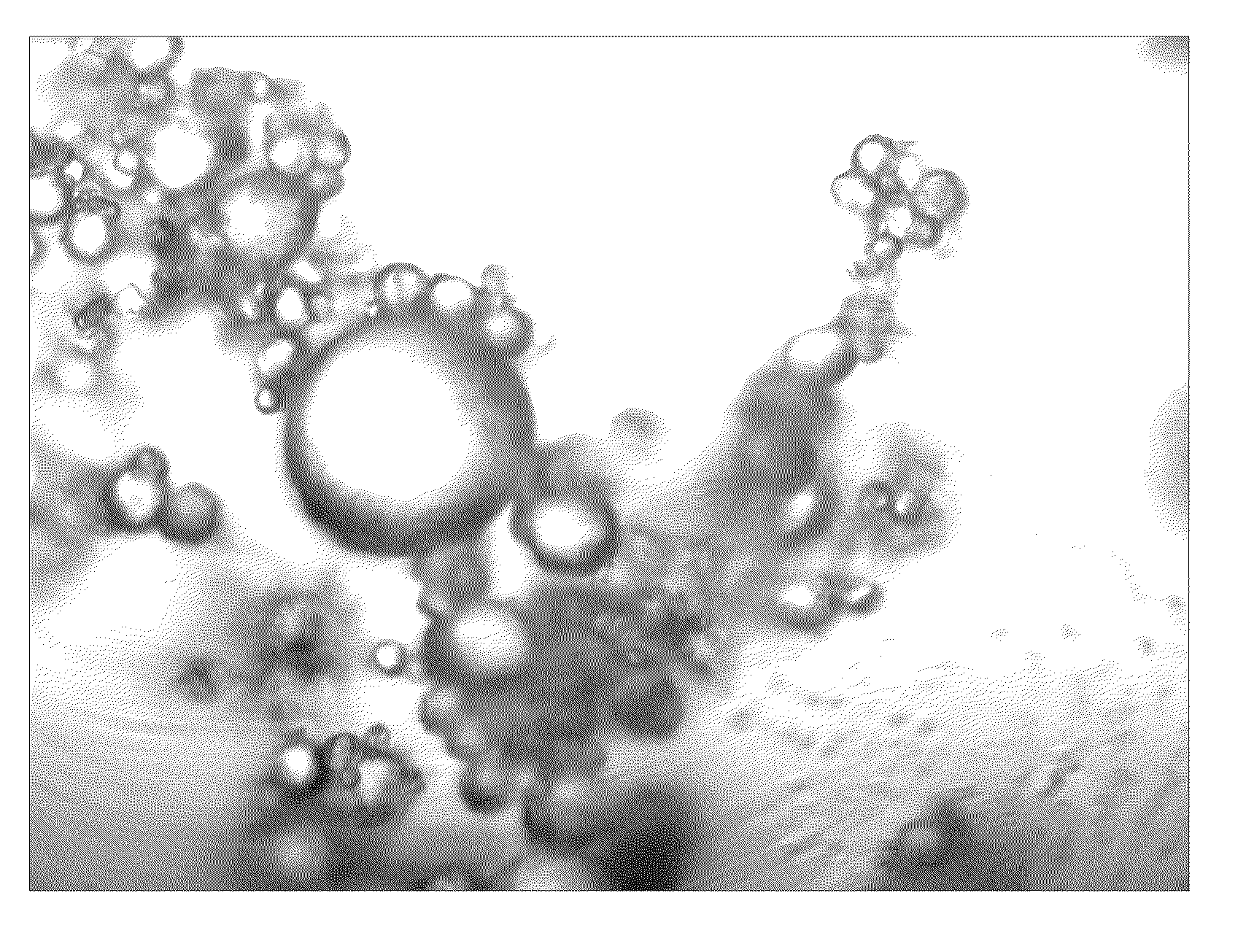
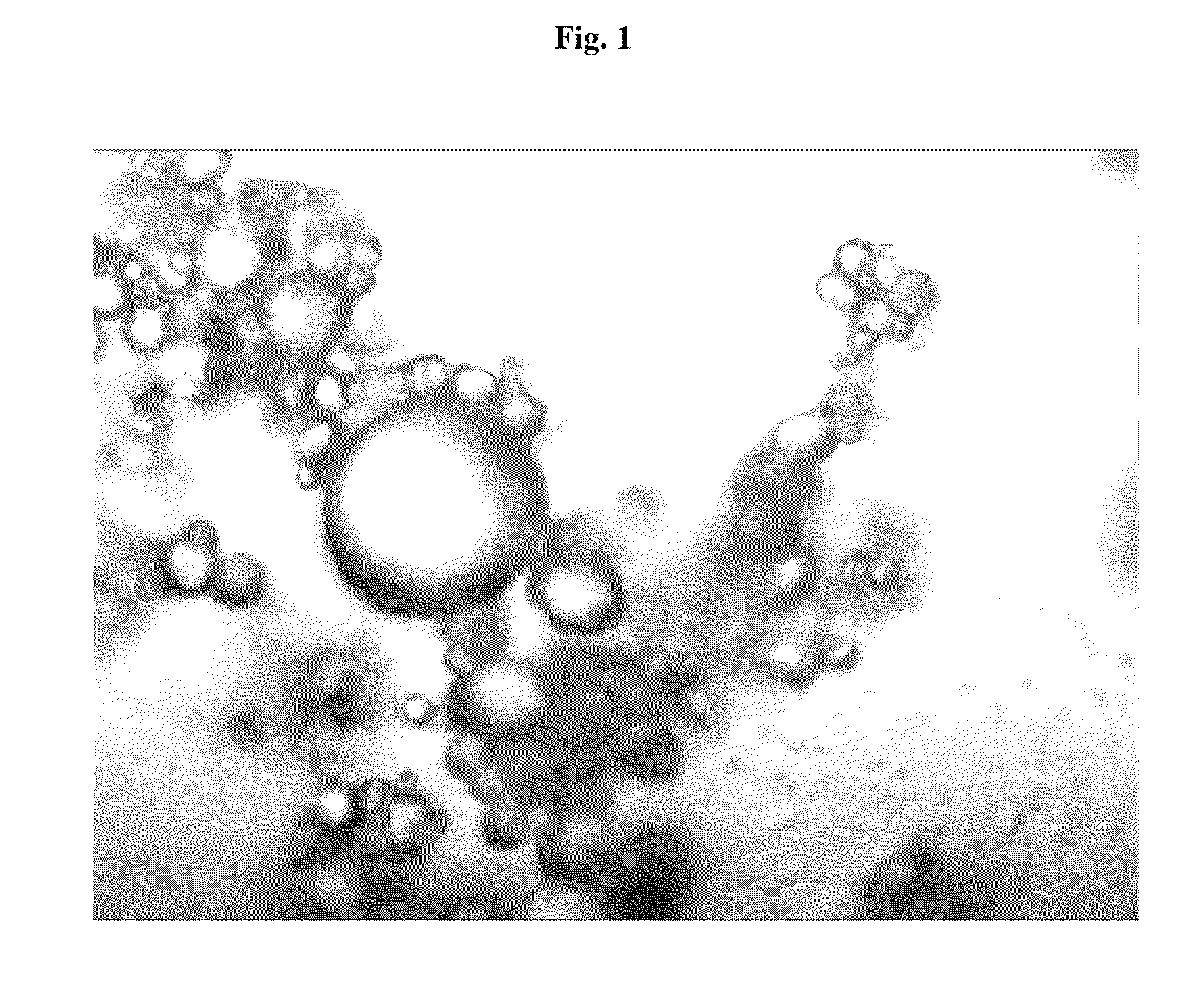
Image
Examples
example 1
[0157]
IngredientsQuantity (g)CoreColesevelam hydrochloride156.250Mannitol20.400Polyvinyl pyrrolidone (Povidone 30)22.500Purified water17.550Isopropyl alcohol62.500Colloidal silicon dioxide2.200Magnesium stearate1.100Film-coatingReady mix film coating material8.800Total228.800
[0158]Procedure: Colesevelam hydrochloride was co-sifted with mannitol (Pearlitol SD 200) using 20# stainless steel sieve and transferred into a rapid mixer granulator and mixed for 5 minutes at 100 rpm. Wet granulation solution was prepared by dissolving povidone in a mixture of isopropyl alcohol and water. Wet granulation solution was added to the Colesevelam-mannitol mixture and mixed at impeller high speed 180 to 200 rpm with chopper off condition for sufficient time till a cohesive mass was formed. The mass was air dried for sufficient time in Glatt drier and further dried at temperature of 50° C. to 60° C. till loss on drying value of about 8% to 12% was achieved. The dried granules were sifted through 100...
example 2
[0159]
IngredientsQuantity (g)CoreColesevelam hydrochloride625.000Mannitol81.600Polyvinyl pyrrolidone (Povidone 30)90.000Purified water70.200Isopropyl alcohol234.800Colloidal silicon dioxide8.800Magnesium stearate4.400Film-coatingReady mix film coating material35.200Total915.200
[0160]Procedure: Colesevelam hydrochloride was co-sifted with mannitol (Pearlitol SD 200) using 20# S. S. Sieve and transferred into Uniglatt fluid bed processor and mixed for 10 minutes. Wet granulation solution was prepared by dissolving povidone in a mixture of isopropyl alcohol and water. The wet granulation solution was sprayed on to the Colesevelam-mannitol mixture in the Uniglatt by top spray mechanism. The mass was dried at temperature of 50° C. to 60° C. for sufficient time till loss on drying value of about 8% to 12% was achieved. The dried granules were sifted through 100# Sieve. Over sized granules were milled using ball mill and the milled mass was sifted through 100# sieve. The sifted granules we...
example 3
[0161]
IngredientsQuantity (g)CoreColesevelam hydrochloride156.250Mannitol25.557Purified water13.980Ethyl cellulose13.813Isopropyl alcohol55.250Colloidal silicon dioxide2.125Magnesium stearate1.063Film-coatingReady mix film coating material8.800Total221.300
[0162]Procedure: Colesevelam hydrochloride was co-sifted with mannitol (Pearlitol SD 200) using 20# S. S. Sieve and transferred into a rapid mixer granulator and mixed for 5 minutes at 100 rpm. Water was added into the rapid mixer granulator and mixed for about 3 minutes at impeller speed of 100 rpm. Wet granulation solution was prepared by dissolving ethyl cellulose in isopropyl alcohol by warming at about 45°-50° C. The binder solution at 45°-50° C. was added to the Colesevelam-mannitol mixture and mixed at impeller high speed 180 to 200 rpm with chopper off condition for sufficient time till a cohesive mass is formed. The mass was air dried for sufficient time in Glatt drier and further dried at temperature of 50 to 60° C. till ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com