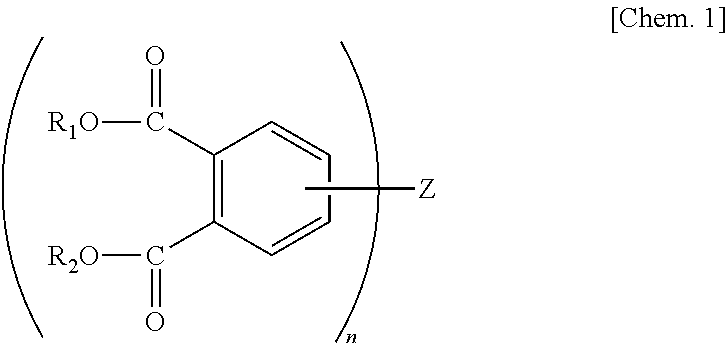
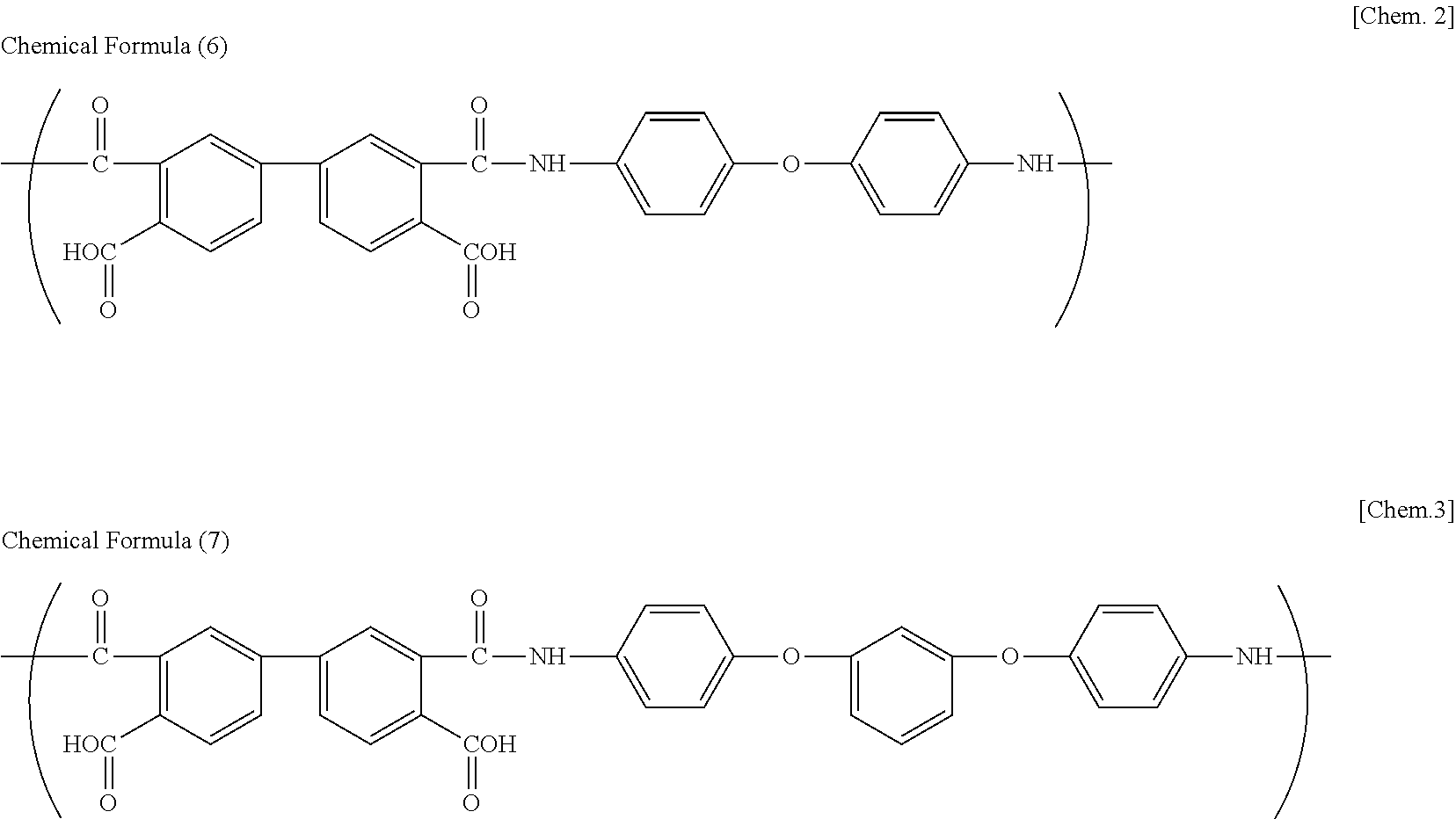
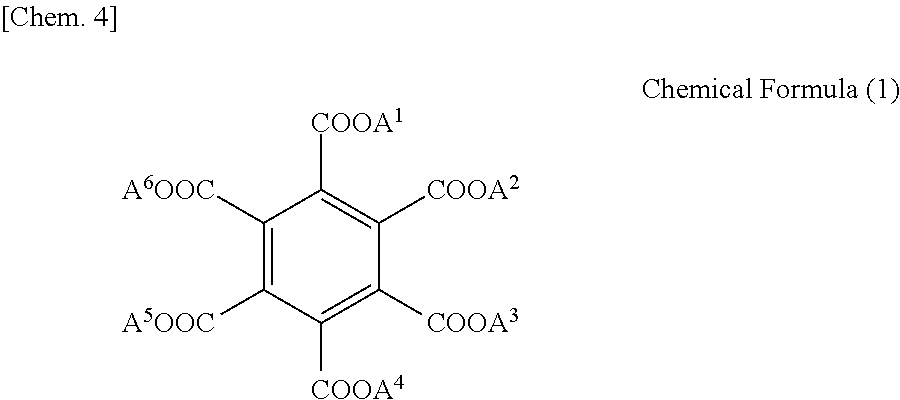
Polyimide precursor solution composition
a technology of polyimide and solution composition, which is applied in the direction of electrode manufacturing process, cell components, coatings, etc., can solve the problems of insufficient properties, failure to disclose mechanical properties, and difficulty in producing satisfactory quality polyimide-resin-formed products, etc., to achieve stable solution viscosity, stable solution viscosity, and the effect of equal or superior properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0122]To 221 g of N-methyl-2-pyrrolidone (also abbreviated hereinafter as “NMP”) was dissolved 20.02 g (0.100 mol) of 4,4′-diaminodiphenyl ether (also abbreviated hereinafter as “ODA”). To this solution was added 28.54 g (0.097 mol) of 3,3′,4,4′-biphenyltetracarboxylic dianhydride (also abbreviated hereinafter as “s-BPDA”), and the mixture was stirred for 24 hours at 25° C. until the s-BPDA dissolved and the solution became viscous, to obtain a solution of polyamic acid (A). The concentration of the polyamic acid in the solution was 18% by mass and the solution viscosity was 41 poise. To this solution was added 0.68 g (0.002 mol; 0.02 times by mole the number of moles of ODA) of mellitic acid, to prepare a polyimide precursor solution composition. The viscosity of the prepared polyimide precursor solution composition was 41 poise, which hardly changed after storage for 3 days at room temperature.
[0123]The prepared polyimide precursor solution composition was casted onto a glass plat...
example 2
[0128]To 221 g of NMP was dissolved 20.02 g (0.100 mol) of ODA. To this solution was added 28.54 g (0.097 mol) of s-BPDA, and the mixture was stirred for 24 hours at 25° C. until the s-BPDA dissolved and the solution became viscous, to obtain a solution of polyamic acid (A). The concentration of the polyamic acid in the solution was 18% by mass and the solution viscosity was 58 poise. To this solution were added, and dissolved evenly, 0.17 g (0.0005 mol; 0.005 times by mole the number of moles of ODA) of mellitic acid (which is 0.08 times by mole with respect to 0.006 mol which is the number of end amino groups of the polyamic acid if theoretical reaction occurs between ODA and s-BPDA) and 0.82 g (0.00225 mol; 0.0225 times by mole the number of moles of ODA) of s-BPTA, to prepare a polyimide precursor solution composition. The solution viscosity of the prepared polyimide precursor solution composition was 58 poise, which hardly changed after storage for 3 days at room temperature.
[0...
example 3
[0130]A polyimide precursor solution composition was prepared in the same way as in Example 2, except that the amounts of mellitic acid and s-BPTA were changed to 0.34 g (0.001 mol) and 0.54 g (0.0015 mol), respectively. The solution viscosity of the prepared polyimide precursor solution composition was 58 poise, which hardly changed after storage for 3 days at room temperature.
[0131]The prepared polyimide precursor solution composition was casted onto a glass plate serving as a base material and dried with hot air for 30 minutes at 120° C. The dried film was peeled off from the glass plate, fixed in a metal frame, and heated for 10 minutes at 250° C. and for another 10 minutes at 300° C., to produce a 39-μm-thick polyimide film. Another polyimide film was produced by instead heating the dried film for 10 minutes at 250° C. and then for another 10 minutes at 350° C. The properties of the resultant polyimide films are shown in Table 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| glass transition temperatures | aaaaa | aaaaa |
| molar ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com