Pharmaceutical composition for treatment and prevention of cancers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
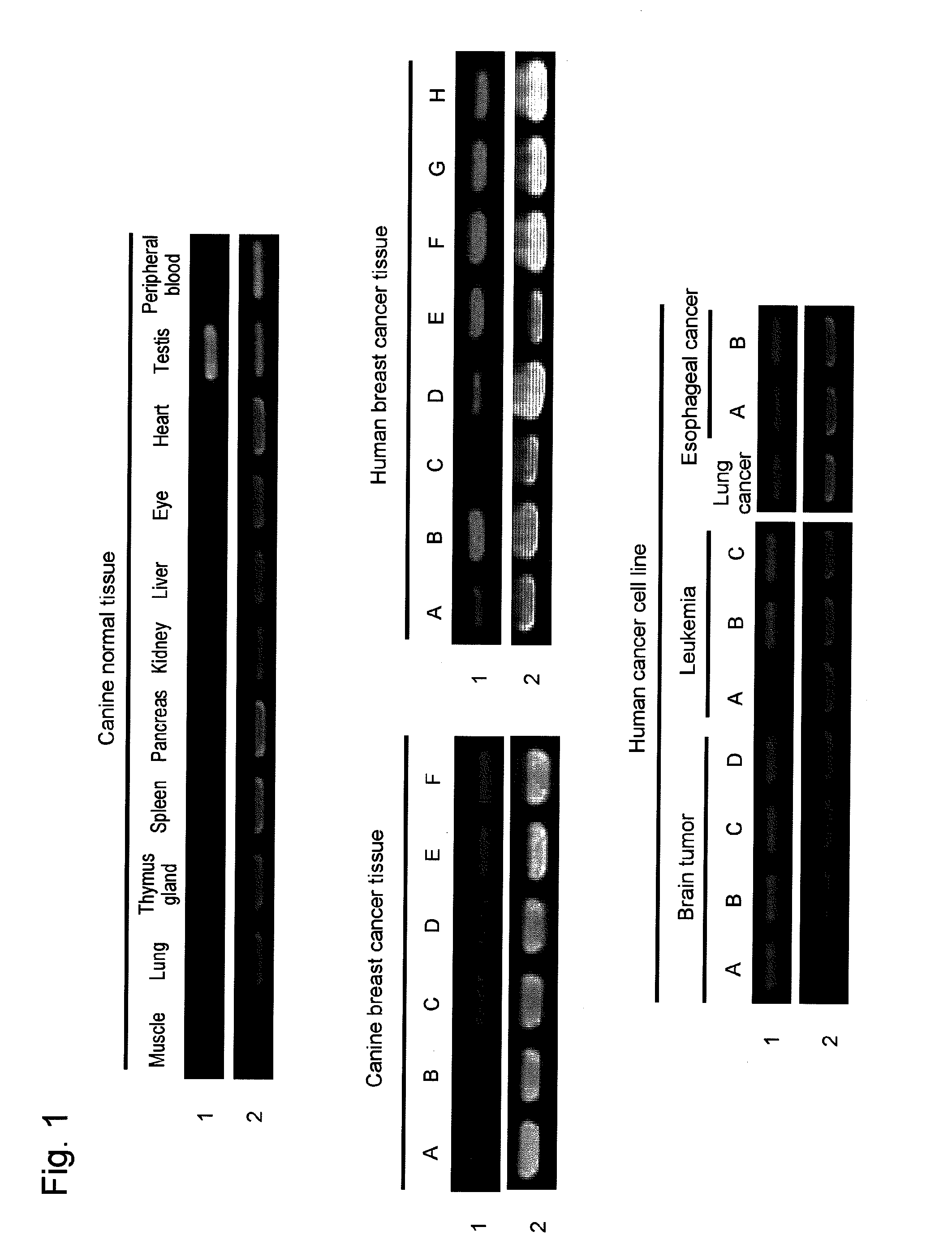
Identification of New Cancer Antigen Protein by SEREX Method
[0155](1) Construction of cDNA Library
[0156]Total RNA was extracted from a testis tissue of a healthy dog by an Acid guanidium-Phenol-Chloroform method and then a polyA RNA was purified according to protocols included with an Oligotex-dT30 mRNA purification Kit (Takara Shuzo Co., Ltd.).
[0157]A canine testis cDNA phage library was synthesized using the thus obtained mRNA (5 μg). The cDNA phage library was constructed using a cDNA Synthesis Kit, a ZAP-cDNA Synthesis Kit, and a ZAP-cDNA GigapackIII Gold Cloning Kit (STRATAGENE) according to protocols included with the kits. The size of the thus constructed cDNA phage library was 7.73×105 pfu / ml.
(2) Screening of cDNA Library using Serum
[0158]Immunoscreening was performed using the above constructed canine testis cDNA phage library. Specifically, host Escherichia coli (XL1-Blue MRF’) was infected with the phage on an NZY agarose plate (Φ90×15 mm) so as to obtain 2210 clones. E. ...
example 2
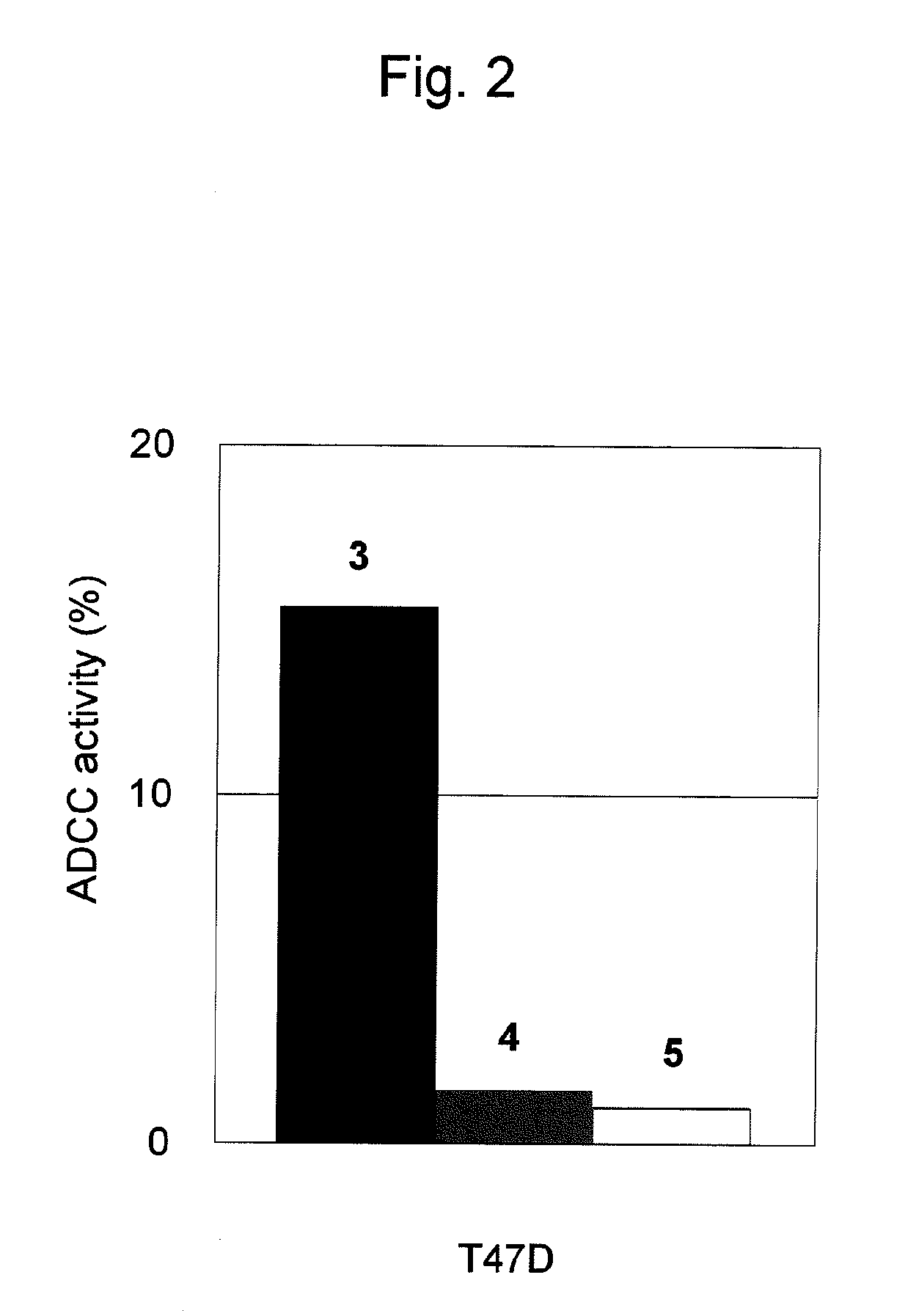
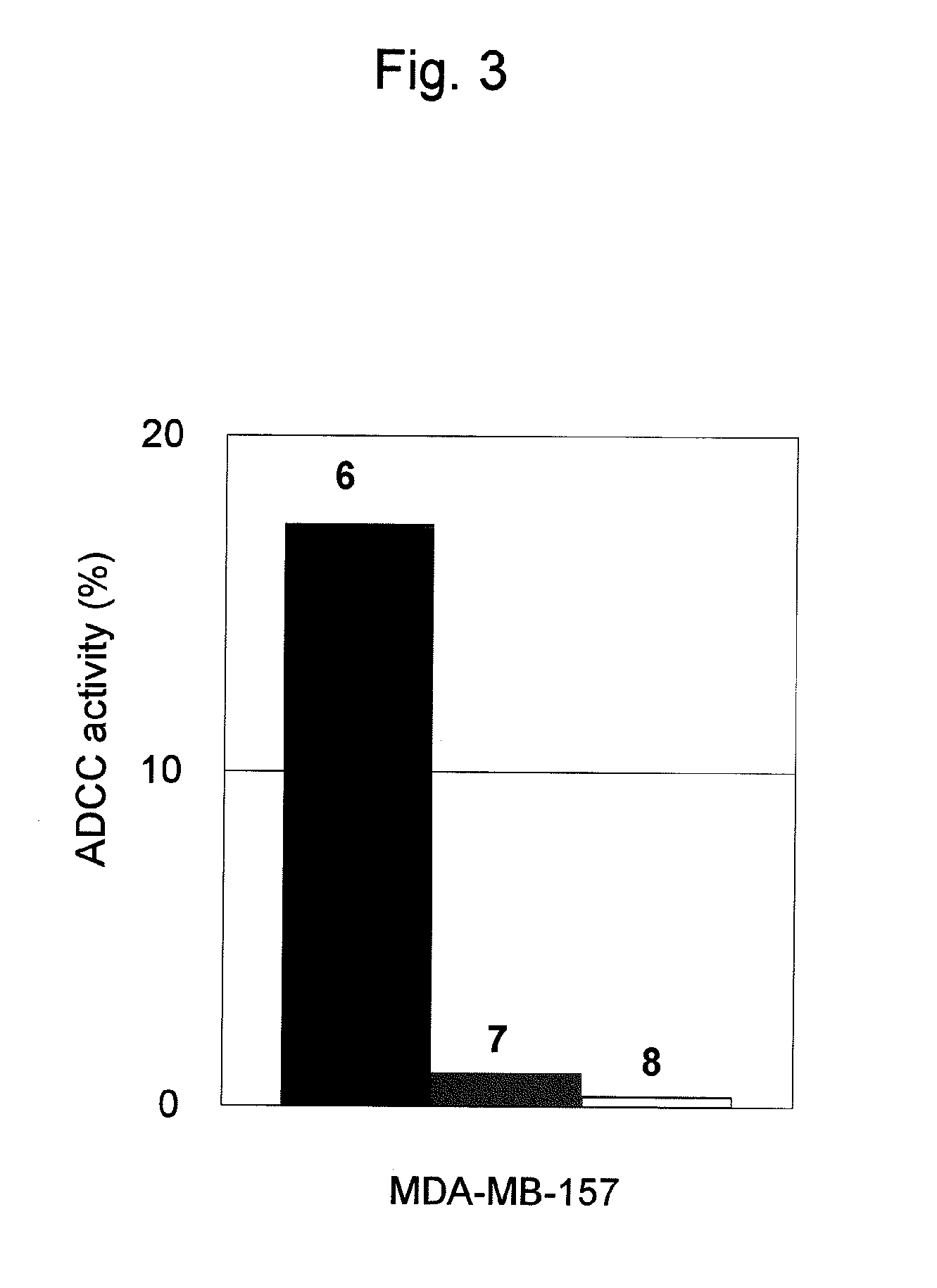
Antitumor Effects (ADCC Activity) of Antibody against CAPRIN-1 upon Cancer Cells
[0172]Next, it was examined whether or not an antibody against CAPRIN-1 would be able to damage CAPRIN-1-expressing tumor cells. Evaluation was carried out using the polyclonal antibody against a human CAPRIN-1-derived peptide prepared in Example 1. Two types of human breast cancer cell lines (T47D and MDA-MB-157) (106 cells each), in which CAPRIN-1 expression had been confirmed, were separately collected into a 50-ml centrifugal tube. Chromium 51 (100 μCi) was added thereto, followed by incubation at 37° C. for 2 hours. Thereafter, cells were washed 3 times with an RPMI1640 medium containing 10% fetal calf serum and added to wells (103 cells per well) in 96-well V-bottom plates. The above polyclonal antibody against a human CAPRIN-1-derived peptide was added thereto (1 μm per well). Further, lymphocytes separated from rabbit peripheral blood were added thereto (2×105 cells per well), followed by culture...
example 3
Preparation of New Human Cancer Antigen Proteins
(1) Preparation of Recombinant Protein
[0174]A recombinant protein of a human homolog gene was prepared by the following method based on the gene of SEQ ID NO: 1 obtained in Example 1. PCR was performed by repeating 30 times a cycle of 98° C. / 10 seconds and 68° C. / 2.5 minutes using a Thermal Cycler (BIO RAD) and a reaction solution adjusted to a total amount of 50 μl through addition of each reagent and an attached buffer (1 μl of cDNA (which was from a variety of tissue / cell-derived cDNAs prepared in Example 1 and observed for their expression by RT-PCR), 2 types of primers (0.4 μM each; SEQ ID NOS: 38 and 39) containing Sad and XhoT restriction enzyme cleavage sequences, 0.2 mM dNTP, 1.25 U PrimeSTAR HS polymerase (Takara Shuzo)). The above 2 types of primers were used to amplify the region encoding the full-length amino acid sequence of SEQ ID NO: 2. After PCR, the thus amplified DNA was subjected to 1% agarose gel electrophoresis an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Reactivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com