Compositions and methods for the storage of red blood cells
a technology of red blood cell and composition, applied in the field of compositions and methods for improving the storage of red blood cells, can solve the problems of scientifically tricky preservation, incremental steps to achieve longer storage duration and higher quality re-infused red blood cells, and reduce the percent recovery of the resulting “packed rbc"
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
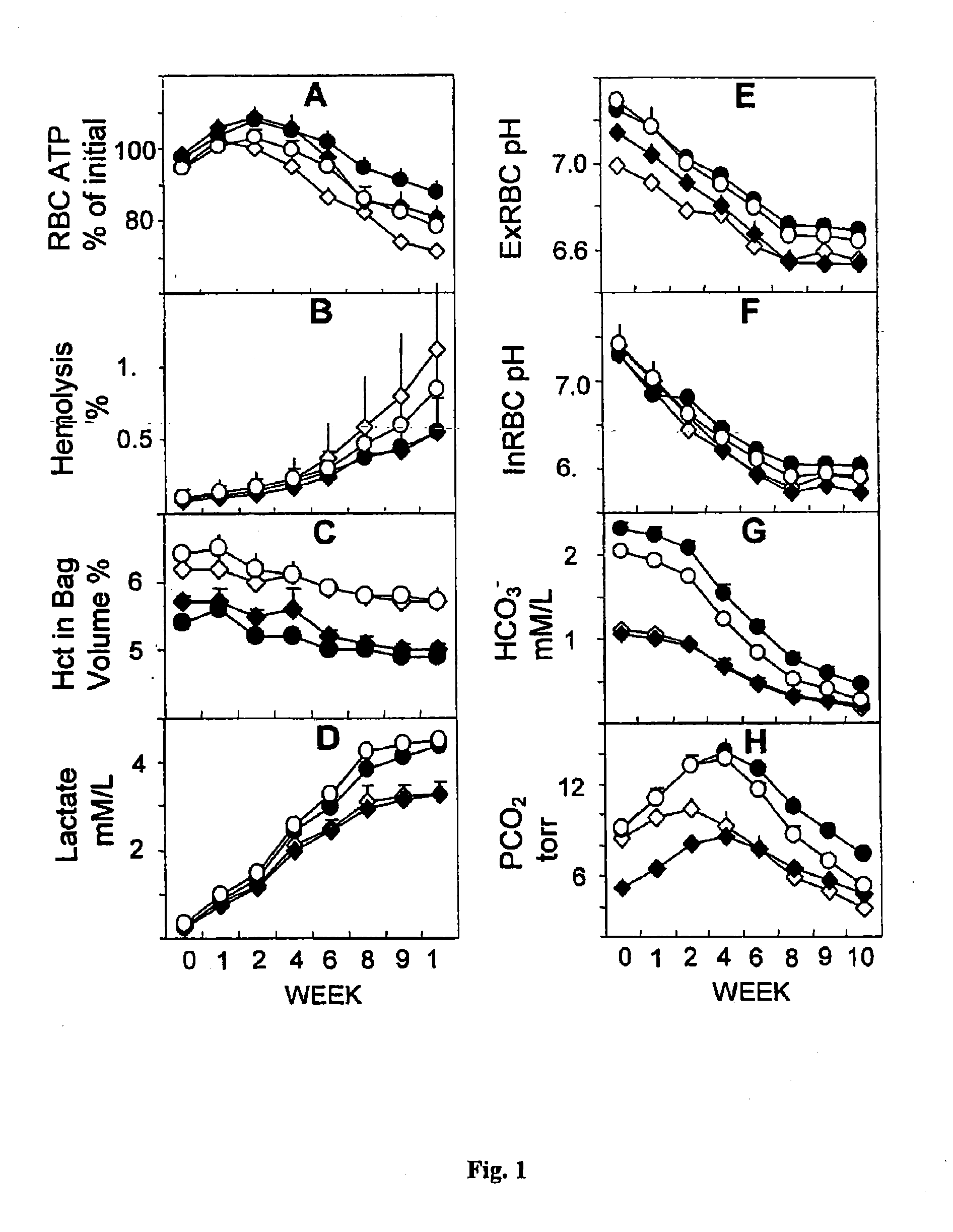
[0063]This example illustrates a performance profile and the advantages of one embodiment of the inventive additive solution composition, designated as EAS-81 (see Table 1). EAS-81 and comparative example AS-3 (Nutricel, Pal Biomedical) are both provided in conventional volumes, while comparative examples EAS-61 and EAS-76v6 are provided in more dilute, larger volumes. EAS-81 and EAS-76v6 both comprise bicarbonate. See FIG. 1, wherein the bicarbonate-containing compositions are represented by circles, while those without bicarbonate are represented by diamonds. Higher volume compositions are represented by solid figures while the conventional volume compositions are represented by open figures. All volumes of additive solution compositions disclosed herein are understood to be per 500 mL unit of whole blood for an approximate volumetric ratio of 1:4.5.
[0064]The first example is conducted as a pooling study in order to evaluate the effect of storage solution ingredients on RBC metabo...
example 2
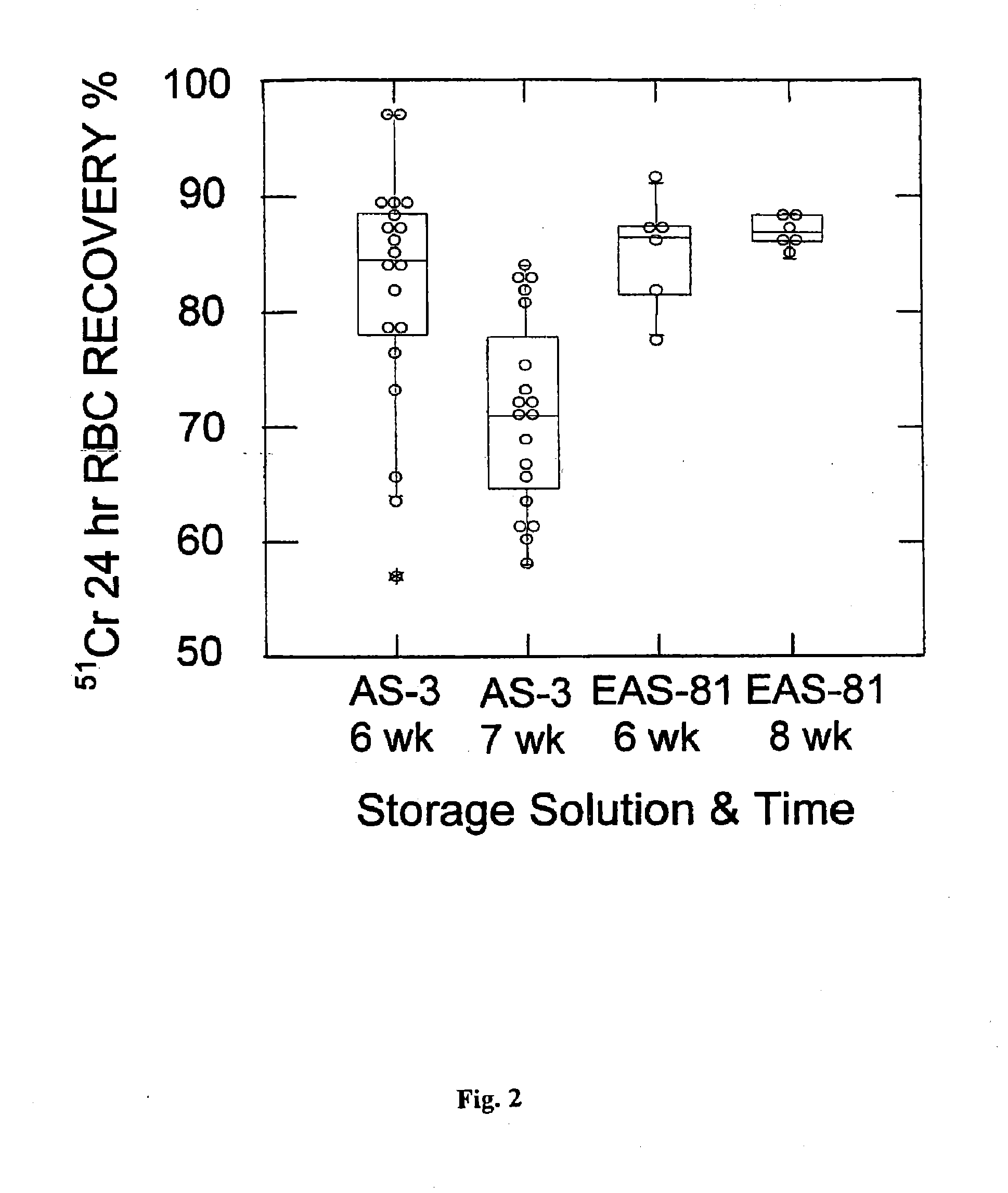
[0073]This example illustrates that an inventive EAS formulation permitted the storage of RBC in conventional-volume additive solution for 8 weeks, with better recovery, lower hemolysis and enhanced membrane preservation over the known 6-week storage solution.
[0074]Twelve volunteer subjects meeting the United States Food and Drug Administration (FDA) and American Association of Blood Banks donor criteria were selected. The subjects donated 500 mL (one unit) of whole blood, which was collected in CP2D primary bag (Item code #127-23, Pall Corporation, East Hills, N.Y.), and leuko-reduced with all but about 65 mL of the plasma removed. EAS-81, 110 mL, was added and the packed RBC solutions were stored upright at 1-6° C. with half the samples (n=6) being stored for 6 weeks and half (n=6) for 8 weeks. The week prior to the end of storage, the units were sterilely sampled and cultured. If the culture exhibited no growth, a small aliquot of the stored RBC was labeled with 51-Cr and returne...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com