Pharmaceutically acceptable salt and polymorphic forms of flupirtine maleate
a technology of flupirtine maleate and polymorphic forms, which is applied in the field of new polymorphic forms of flupirtine maleate, can solve the problems of affecting the quality, safety and efficacy of a drug product, and achieve the effect of improving the safety and efficacy of the drug produ
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
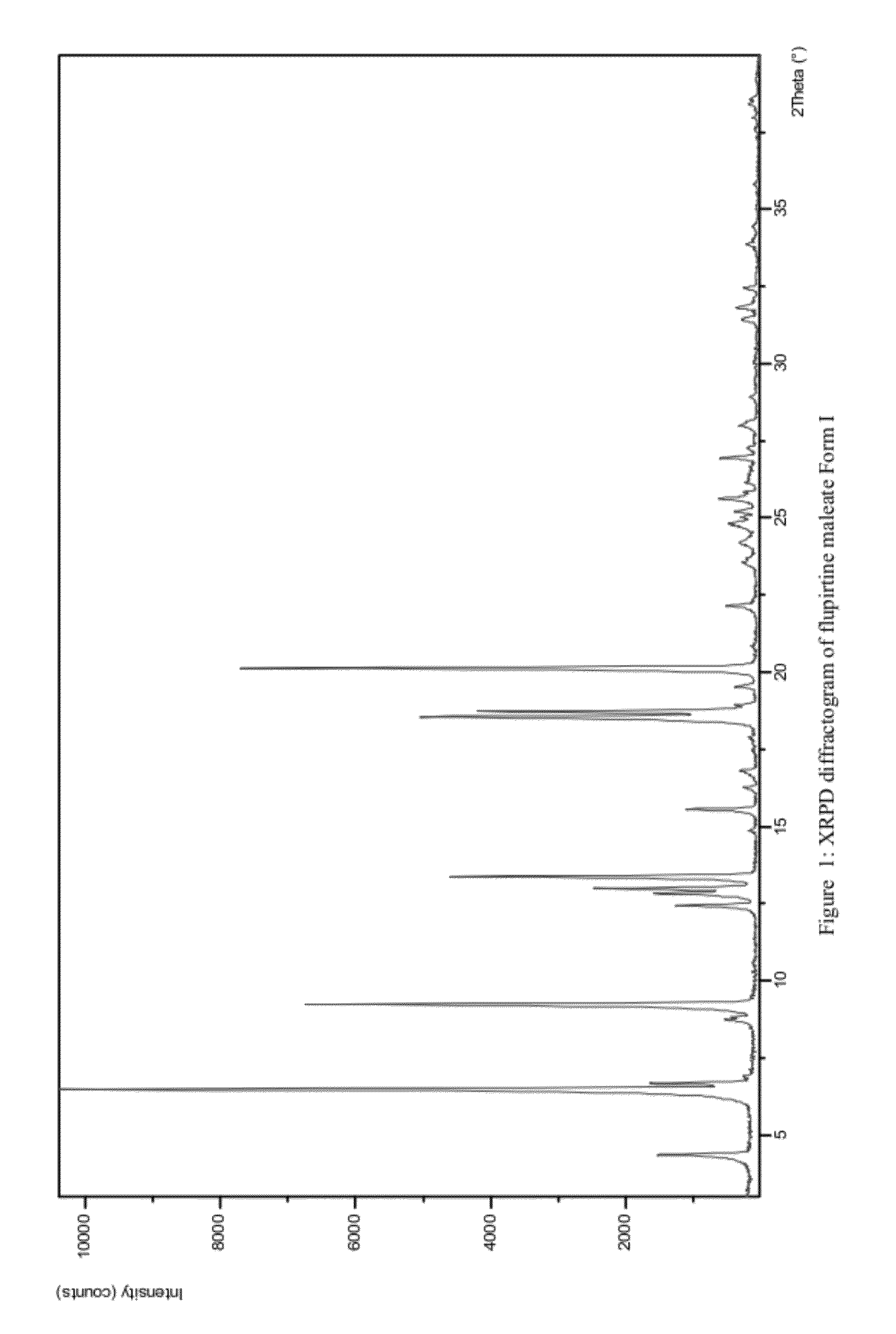
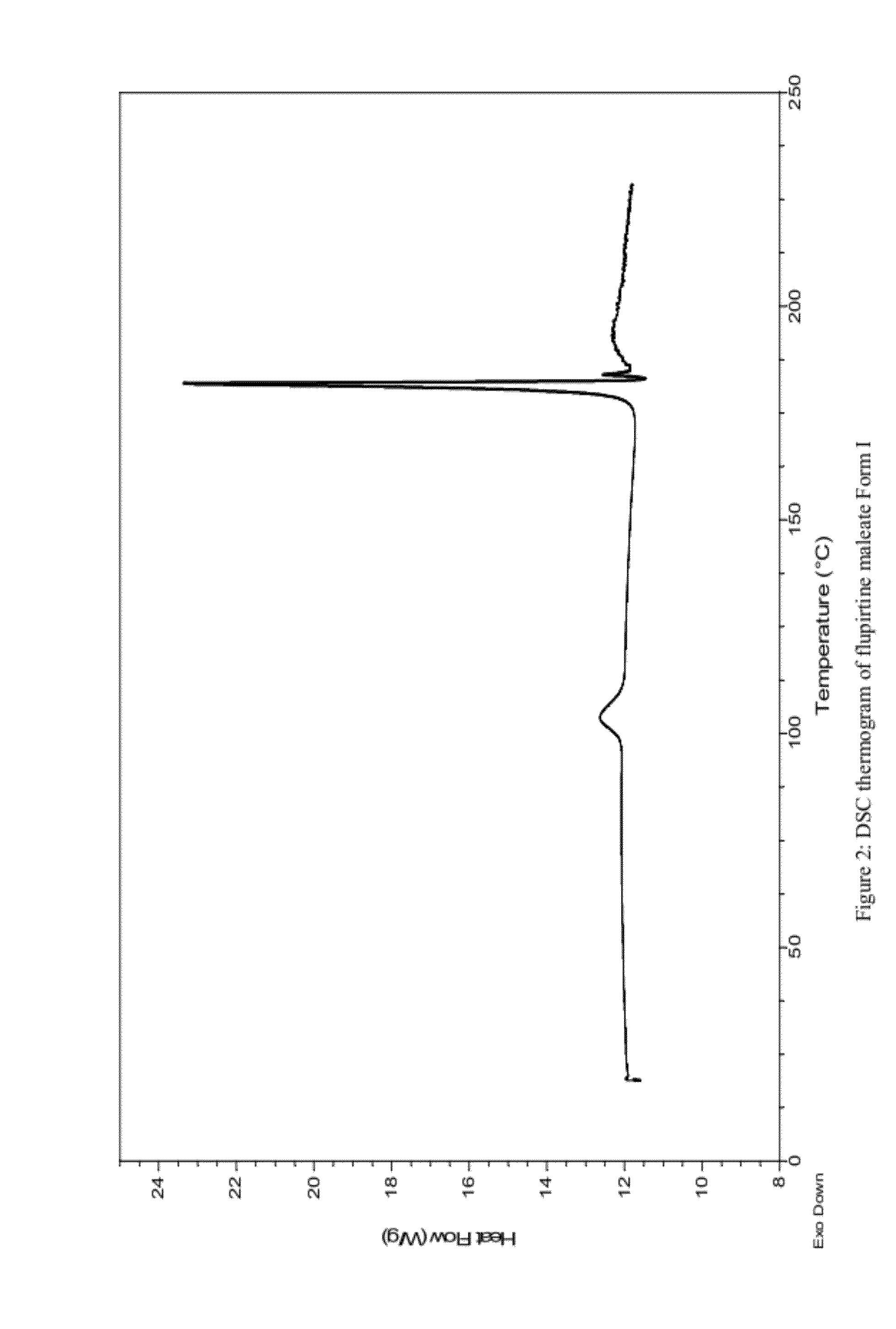
Preparation of Flupirtine Maleate Form I
[0071]250 mg of flupirtine maleate was dissolved in 35 mL of methyl acetate whilst heating. Hot solution was filtered and crystallisation of needle like crystals occurred while cooling the solution to room temperature. The product was collected by filtration and dried overnight at room temperature. 136 mg of a white crystalline product was obtained.
example 2
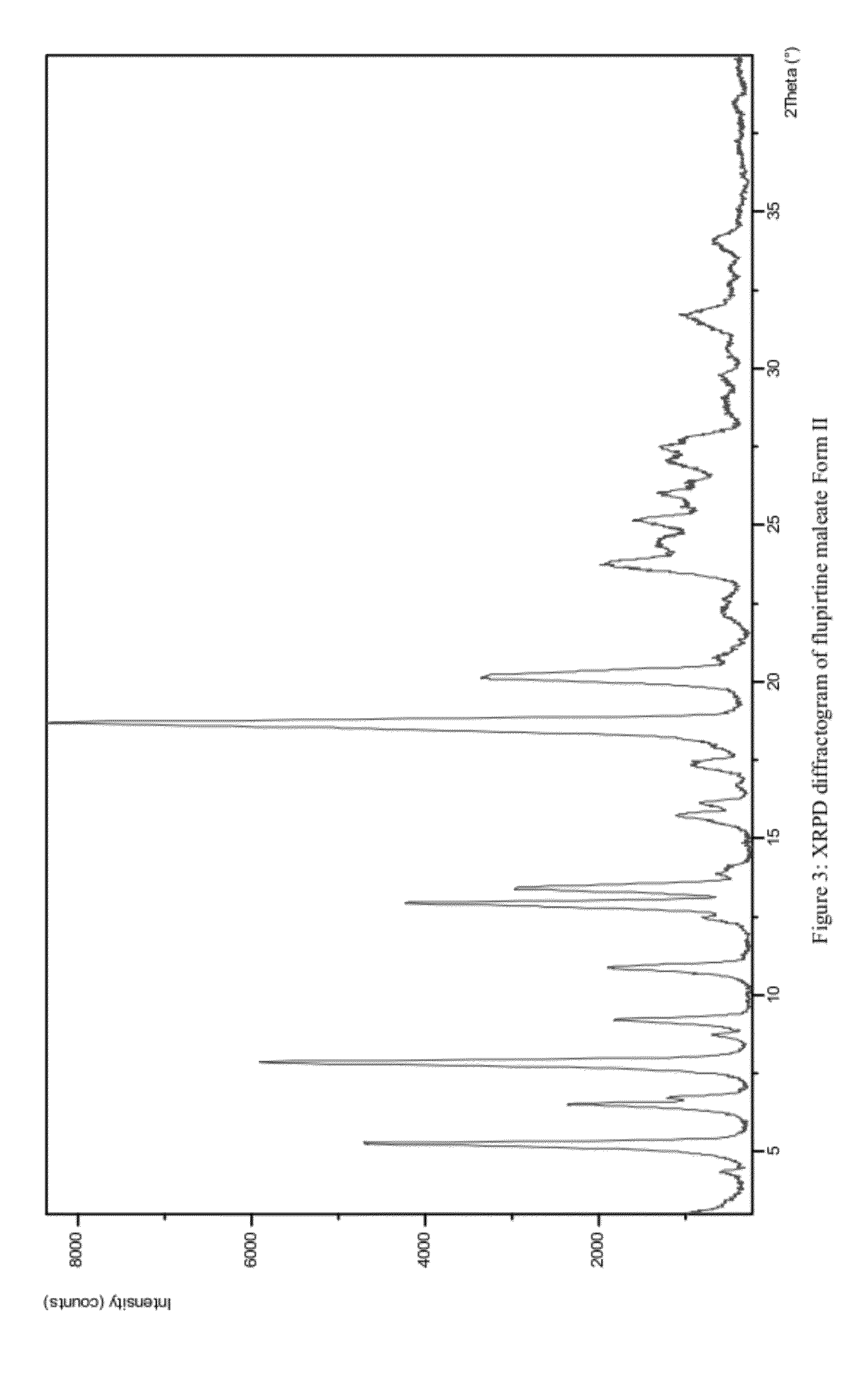
Preparation of Flupirtine Maleate Form II
[0072]500 mg of flupirtine maleate was placed in 3-neck round bottom flask filled with a mixture of 96% ethanol (15 mL) and dichloromethane (30 mL). The reaction mixture was refluxed for 20 minutes whilst stirring and then cooled in an ice bath to room temperature. Stirring was then continued at room temperature overnight. The product was filtered and dried overnight at room temperature. 314 mg of a white product was obtained.
example 3
Preparation of Flupirtine Maleate Form III
[0073]500 mg of flupirtine maleate was dissolved in 10 mL of 96% ethanol. The solution was heated and the hot solution was filtered and added dropwise to 20 mL of hot methyl acetate. The mixture was stirred at room temperature and precipitation of a crystalline product occurred. The product was filtered. 320 mg of a white product was obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Angle | aaaaa | aaaaa |
| Angle | aaaaa | aaaaa |
| Angle | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com