Medical use of spla2 hydrolysable liposomes
a technology of liposomes and liposomes, which is applied in the direction of drug compositions, peptide/protein ingredients, biocide, etc., can solve the problems of increased efficacy and death of mice, and achieve the effects of prolonging the therapeutic effect reducing the side effects of the therapeutic agent, and reducing neurotoxicity and gastrointestinal toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of sPLA2 Liposomes (LiPlaCis)
[0101]A lipid intermediate is prepared by spray-drying the following a mixture of phospholipids (70 / 25 / 5 mol % DSPC / DSPG / DSPE-PEG2000). The lipids are dissolved in methanol and chloroform. The lipid intermediate is hydrated in an aqueous solution of the anti-cancer drug with agitation. At this step the liposomes are formed but they have a broad size distribution and is a mixture of single-layer and multiple-layer liposomes. In order to get a product with a narrow size distribution and mono-layer liposomes the hydration mixture is extruded by passing it through poly-carbonate filters of appropriate pore sizes. To remove un-encapsulated anti-cancer drug the mixture is purified. A number of techniques are available e.g. dialysis, gel-filfration and ultra-filtration. For preparations ranging from a few liters and above ultra-filtration is the preferred method. Preparations intended for parenteral administration must be sterilized e.g. by sterile-...
example 2
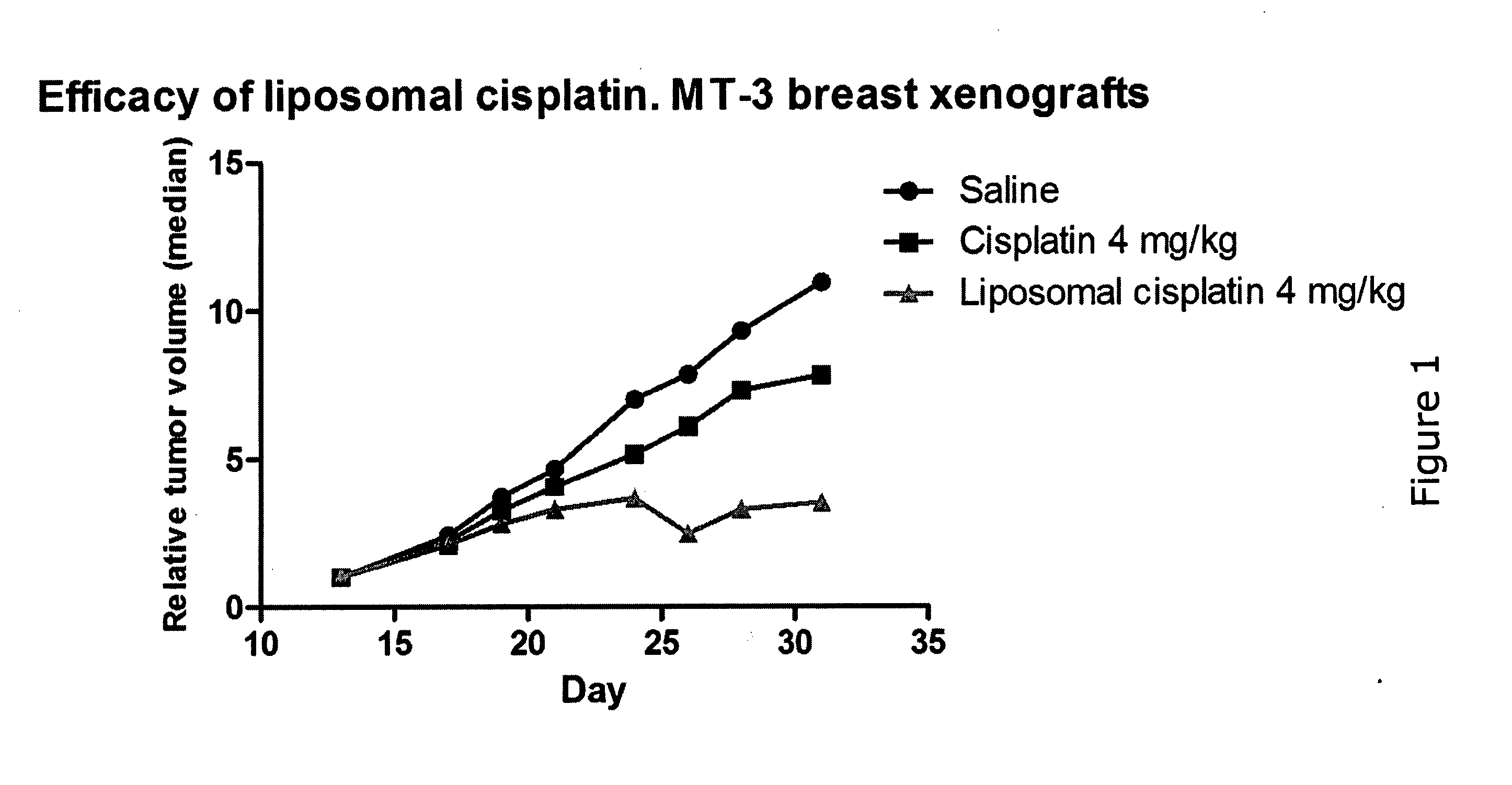
Efficacy in Mice
Methods
[0102]NMRI nude female mice (6-8 weeks) were inoculated subcutaneously into the left flank with 1*107 cells of the human breast carcinoma cell line MT-3. Only mice carrying exponentially growing tumors were selected for the study. Treatment started when tumors had reached a size of 70-80 mm3. Animals received one dose (4 mg / kg cisplatin (Platinol), LiPlaCis or saline) weekly with intra-venous injections into the tail vein starting on day 13 after tumor transplant. Tumor growth was assessed three times a week by measuring two perpendicular diameters and tumor growth was normalized for differing starting sizes by calculating relative tumor volume. Body weight was measured three times a week. Blood samples were taken four days after the first injection to estimate white blood cells and thrombocytes by Coulter counter.
Results:
[0103]LiPlaCis was compared with cisplatin and saline in an efficacy study using MT-3 breast xenografts on nude mice. Cisplatin and LiPlaCis...
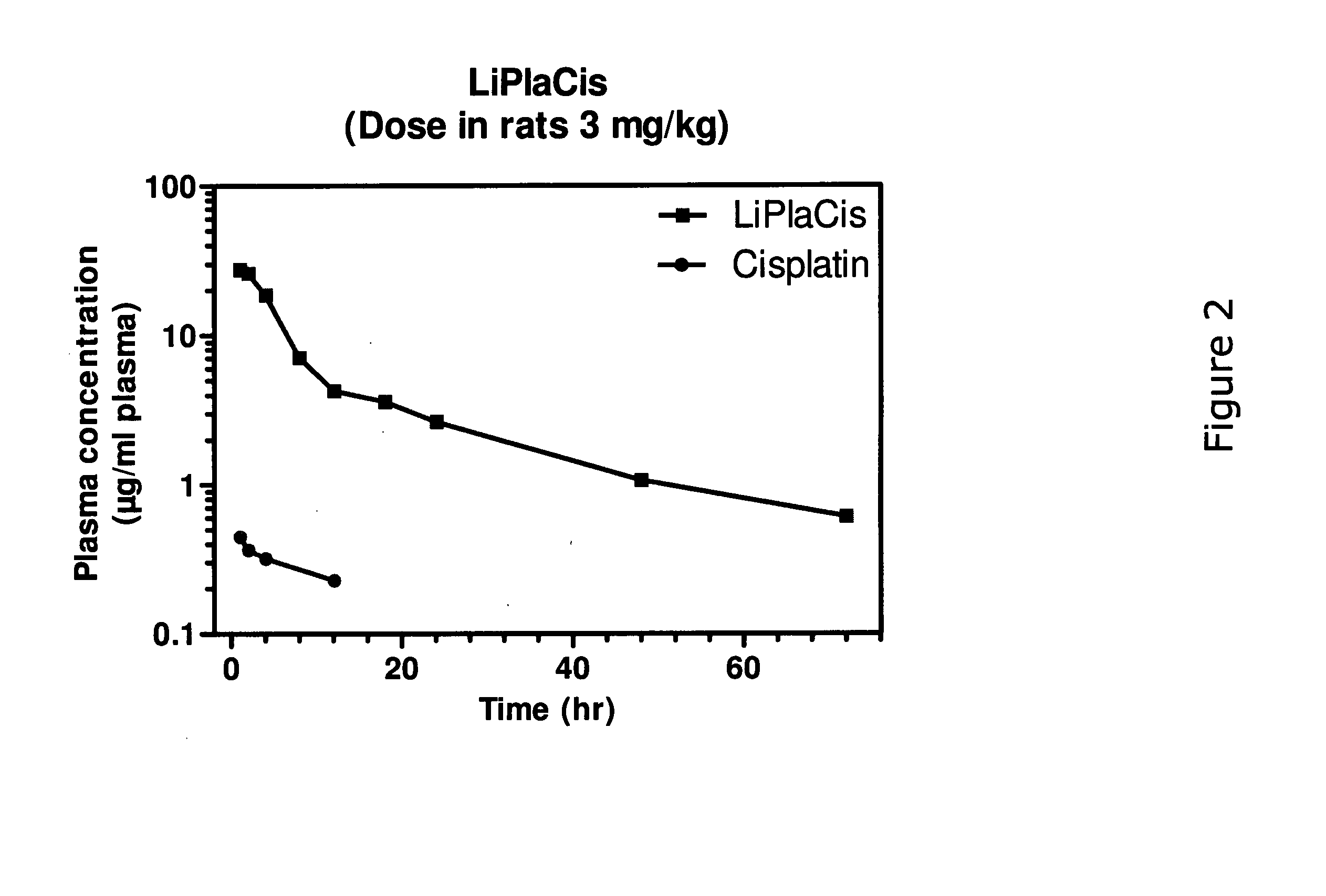
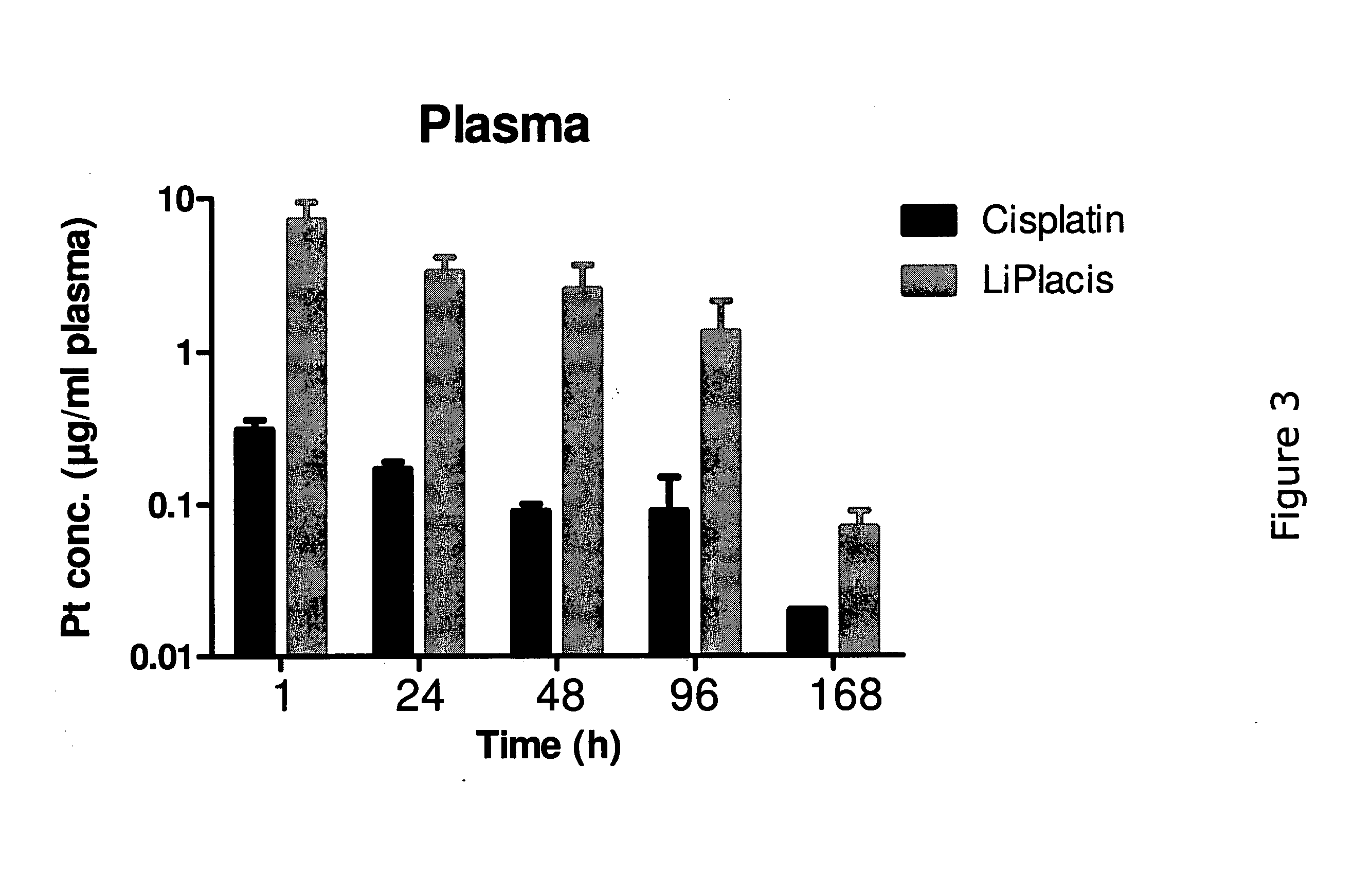
example 3
Pharmacokinetics in Rats
Methods:
[0104]Rats (BrlHan:WIST@Mol(GALAS)) were injected with 3 mg / kg cisplatin or LiPlaCis and blood was collected into heparinised tubes (Microvette CB 300 Sarsted). Samples were taken from 10 minutes up to 72 h. A blood volume of 250 μl was taken from each sampling point and immediately placed in an ice-bath and centrifuged (3000×g; 5 min) to obtain the plasma fraction. The plasma-containing tubes were frozen until shipment and subsequent digestion in HCl / HNO3 / H2O2 (60 / 5 / 35 vol %) before platinum analysis using ICP-MS.
Results and Conclusion:
[0105]The experiment revealed that LiPlaCis a long-circulating liposomal form of cisplatin with a T½ of about 20-23 h compared to the 15 minutes for free cisplatin. The area under the curve (AUC) for LiPlaCis was at least 50 times that of cisplatin. See FIG. 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com