Global nav1.7 knockout mice and uses
a technology of nav1.7 and mice, applied in the field of global nav1.7 knockout mice and uses, can solve the problem of complete inability of otherwise healthy individuals to sense any form of pain, and achieve the effects of preventing flinching behavior, no flinching or licking behavior, and robust flinching behavior
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
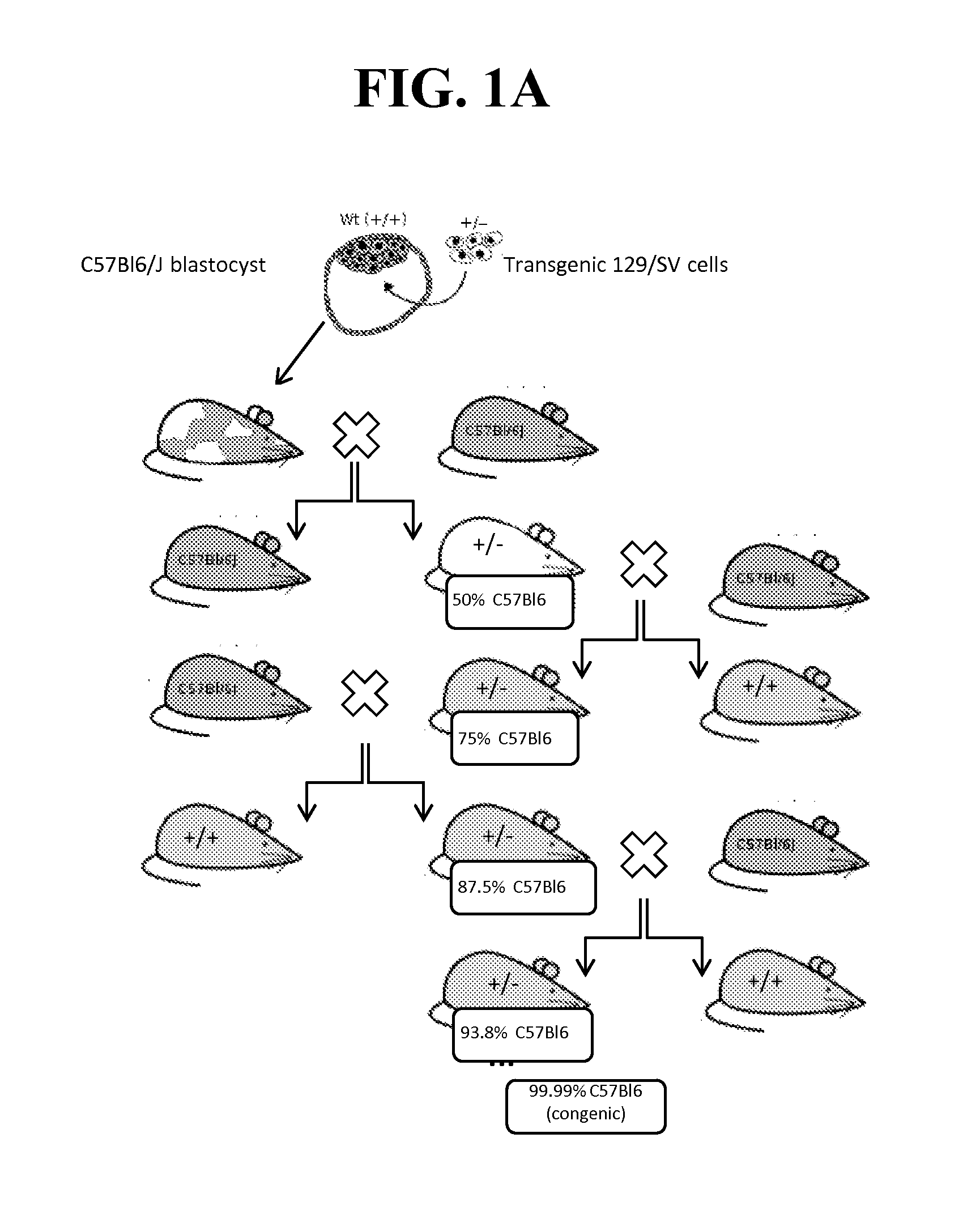
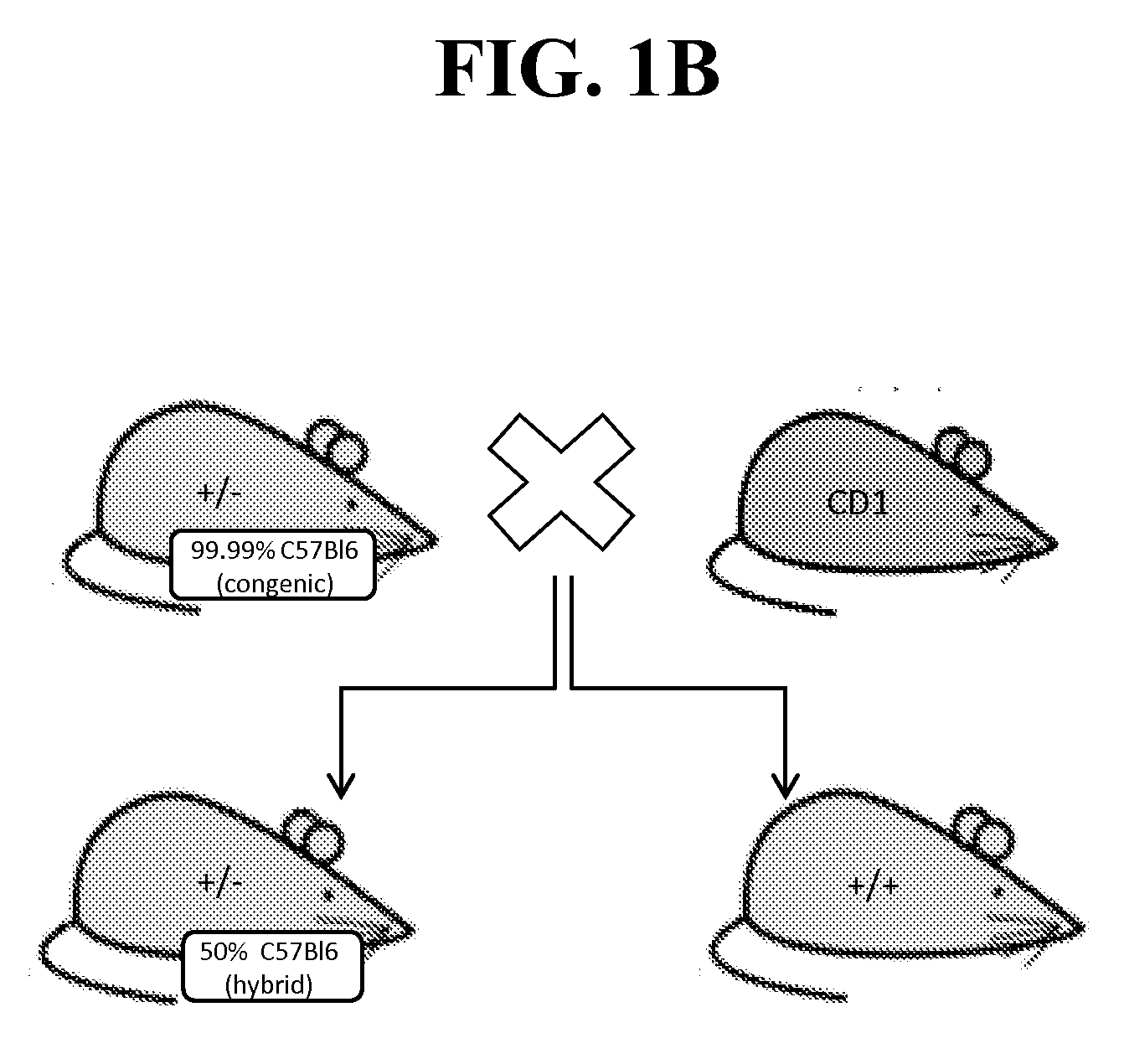
[0231]Because of the reported neonatal lethality of NaV1.7 KO animals (heterozygous Nav1.7+ / − mice from Deltagen, San Mateo, Calif.: B6.129P2-Scn9 atm1Dgen / J backcrossed at least 8 generations to C57BL / 6) due to an apparent failure to feed (Nassar et al., 2004), we outcrossed these animals onto a CD1 background to add vigor to the line, as well as a separate backcross onto a BALB / c line to create a congenic line (first breeding pair received in May 2011). (See, FIG. 1A-B).
[0232]No significant differences were noted in Scn9a-CD1 KO or Scn9a-Balbc KO animals. We refer to these animals as NaV1.7 KO and specify the strains when required. All data displayed herein were collected on single out- and backcross. Further backcrosses (for BALB / c line only) are currently being performed, thus far results obtained were identical on a N4 backcross generation. No further increase in survival was observed. The final outcome of the backcrossing has yet to be determined up...
example 2
Preparation of Artificial Mouse Milk (AMA)
[0245]The procedure and composition of the artificial mouse milk prepared was modeled closely after that described by Yajima and co-workers and by Auestad and co-workers. (Yajima, M, et al., A Chemically Derived Milk Substitute that is Compatible with Mouse Milk for Artificial Rearing of Mouse Pups” Exp. Amin. 55(4), 391-397, (2006); Auestad, N, et al., Milk-substitutes comparable to rat's milk; their preparation, composition and impact on development and metabolism in the artificially reared rat, Br. J. Nutr. 61: 495-518 (1989)).
[0246]Reagents and Procedure. Reagents used, including source, amounts used, solvents and amounts used, and miscellaneous comments are listed in Table 3 (below).
[0247]Procedure. Step 1: Sodium hydroxide was weighed and transferred to a 10-L beaker. Distilled Water (1.3 L) was added and an overhead stirrer was put in place. Potassium hydroxide was weighed and added to this solution, as were L-serine, L-cystine and L-...
example 3
[0250]The following primers were used to genotype all animals in the colony: Forward Scn9a 5′ AGA CTC TGC GTG CTG CTG GCA AAA AC 3′ (SEQ ID NO:1); Reverse Scn9a 5′ CGT GGA AAG ACC TTT GTC CCA CCT G 3′ (SEQ ID NO:2) and Forward Neomycin 5′ GGG CCA GCT CAT TCC TCC CAC TCA T 3′(SEQ ID NO:3). These primers gave rise to an endogenous band of 267 base pairs (Forward Scn9a+Reverse Scn9a) or a targeted band of 389 base pairs (Forward Neomycin+Reverse Scn9a). PCR cycling conditions were as follows: (1) 95° C. for 7 minutes, followed by 35× cycles of (2)-(4) below, followed by (5)-(6):
[0251](2) 96° C. for 10 seconds;
[0252](3) 60° C. for 30 seconds;
[0253](4) 72° C. for 1.5 minutes,
[0254](5) 72° C. for 7 minutes;
[0255](6) cool to 4° C.
[0256]The genotyped patterns were as follows:
[0257]WT or + / +=endogenous (E) band only
[0258]HET or + / −=endogenous (E)+targeted (T) bands
[0259]KO or − / −=targeted (T) band only
[0260]DNA concentrations ranging between ˜25 ng up to 1 μg were originally tested...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| holding voltage | aaaaa | aaaaa |
| repolarization voltage | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com