Synthesis of Degradable Polymers Downhole for Oilfield Applications
a technology of polymerization and oilfield, applied in the direction of fluid removal, wellbore/well accessories, chemistry apparatus and processes, etc., can solve the problems of coiled tubing or surface equipment, reducing the concentration of solids and the velocity of slurry pumping, and difficult control of solid placemen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
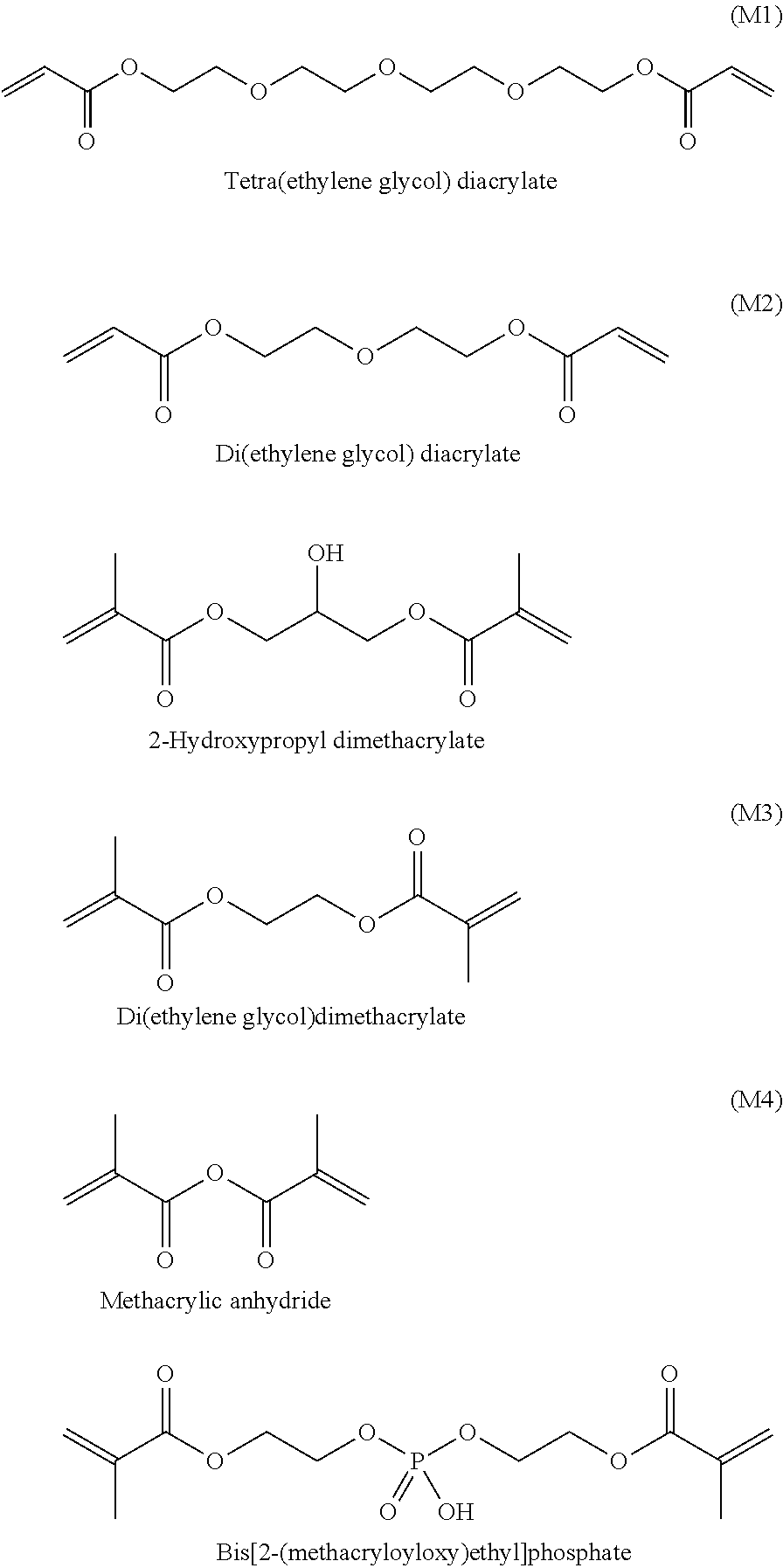
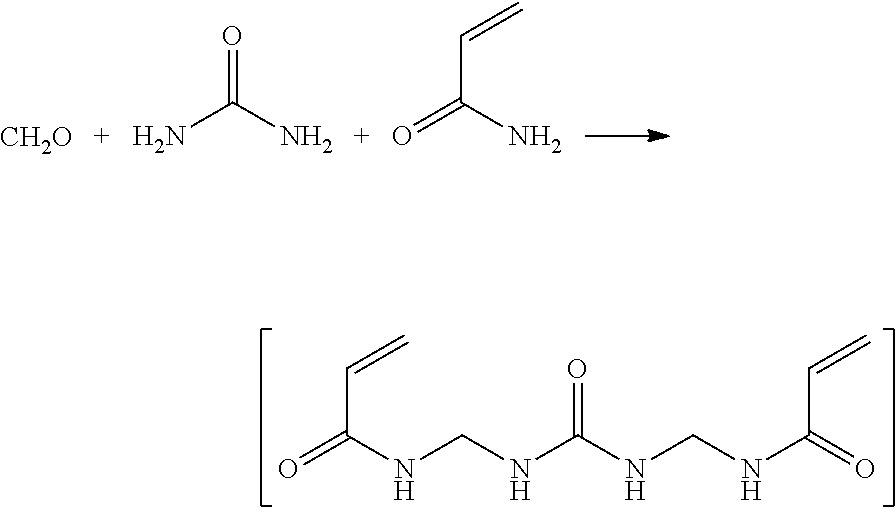
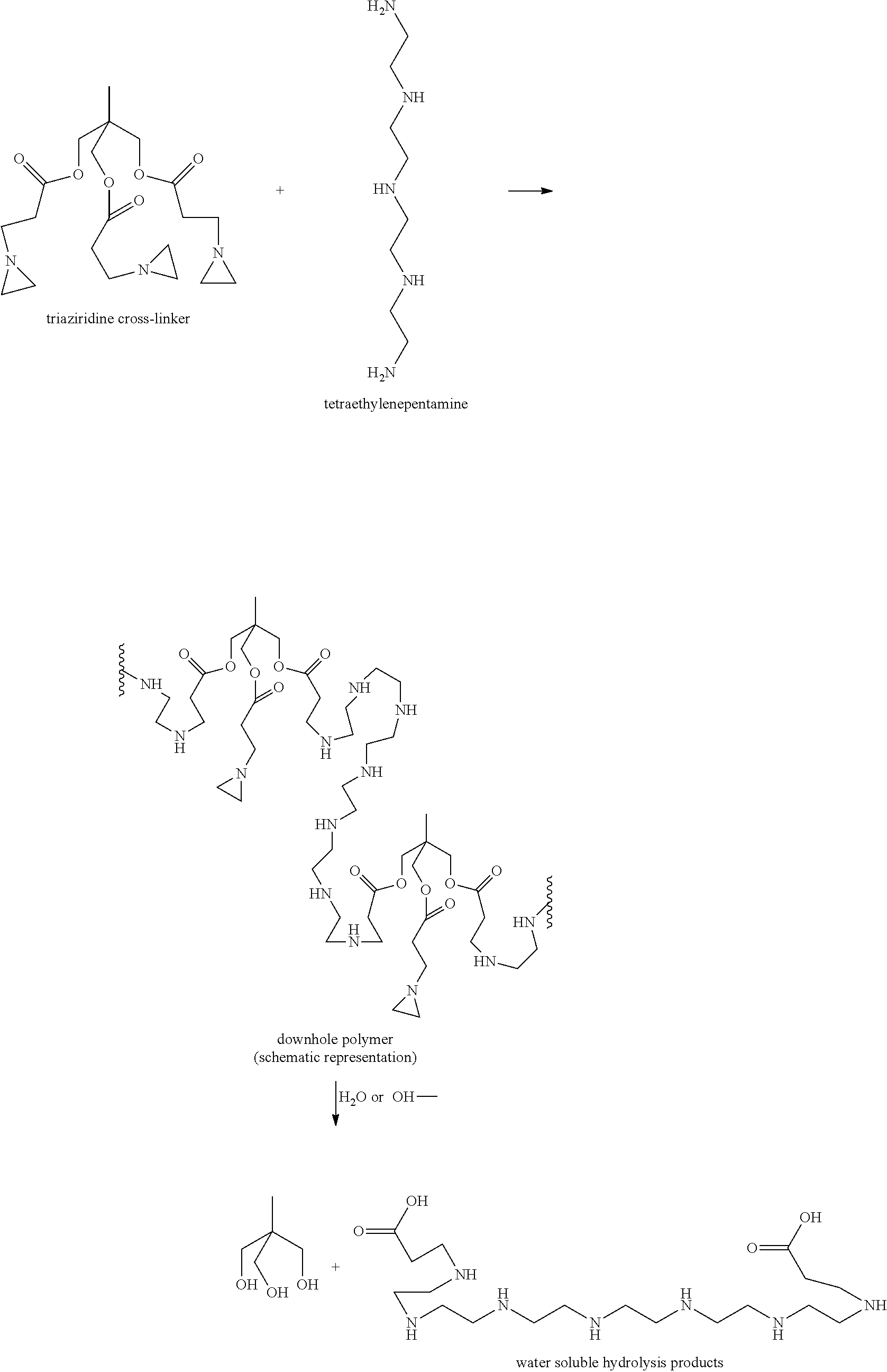
[0057]It is well known that methacrylates cannot undergo thermal self-initiation of polymerization. Thus, for the polymerization of these monomers, radical polymerization initiators were employed. The number of polymeric chains in the polymerization is in direct proportion to the initial concentration of initiator. As polymerization proceeds up to a monomer conversion of 100%, then for a given polymer concentration, the larger the initial initiator concentration, the larger the number of growing polymer radicals that will be formed and be able to grow; thus there is a smaller number of monomeric units in each polymer chain. Thus, by varying the initiator / polymer concentration ratio, one can control the polymer molecular weight, and therefore the ease of polymer hydrolysis.
[0058]Polymerization of M1 in the presence of 10% of ammonium persulfate as the initiator was performed by the standard procedure described above, yielding white flakes of dry polymer. Hydrolysis of the polymer was...
example 2
[0059]Samples containing 0.5 M monomer in 5 ml of water were prepared with double the critical micelle concentration of surfactant (0.015 M for SDS and 0.007 M for TTAB) and treated with an Ika-Werke T 10 basic emulsifier for 5 minutes. The stability of the emulsions vs. time was checked; the results are sown in Table 1:
TABLE 1M1M2M4timeSDSTTABSDSTTABSDSTTAB5minStableStableStableStableStableStable10minStableStableStableStableStableStable20minStableStableAA——30min————BB1hourBBBBBB2hoursBBBBCB5hours————CB6hours————D24hoursEFCGGGA: White precipitate formed as emulsion begins to separate and disappears after mixing; emulsion was white.B: White precipitate which disappeared after mixing; solution was white.C: White precipitate which disappeared after mixing; solution was transparent.D: Emulsion nearly completely separated.E: Emulsion started to separate.F: Emulsion stared to separate; less stable than E (with SDS).G: White precipitate which disappeared after mixing; solution was transpar...
example 3
[0060]Emulsion polymerization experiments were carried out as follows: Samples containing 0.5 M monomer in 5 ml of de-ionized water were prepared with double the critical micelle concentration of surfactant (0.015 M (0.0217 g / 5 ml) for SDS and 0.007 M 0.0118 g / 5 ml) for TTAB). The mixture was stirred with an emulsifier for 5 mm. Ammonium persulfate initiator (2.5 10−3 mol / l (0.0029 g / 5 ml)) was added. The mixture was degassed by argon bubbling for 10 min and then heated in an oil bath to 60° C. for about 10 min to about 7 hours, depending on the monomer. The polymers obtained were dried at 100° C. The results are shown in Table 2.
TABLE 2M1M2M4SDSTTABSDSTTABSDSTTABTime of polymerization556.56.5Rapid*Rapid*hourshourshourshoursPhysicalClottedClottedCloudyCloudyCloudyCloudystate ofwhitewhitesolu-solution withsolu-solutionthepolymerpolymertionmaterialtionwithpolymerinsoluble,glass-in waterlikedropletsparticles*Mixture polymerized during bubbling of argon in less than 5 min.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com