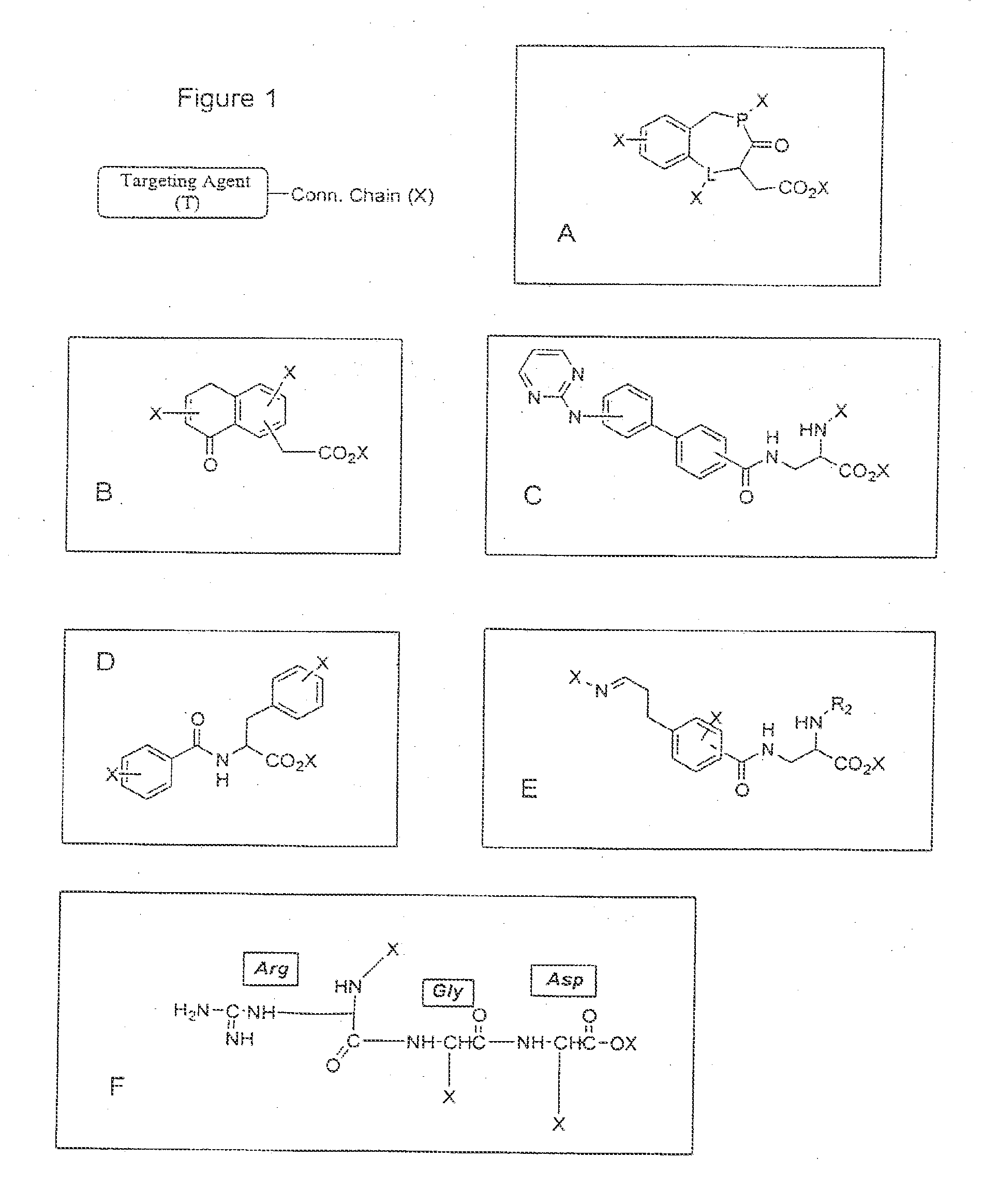
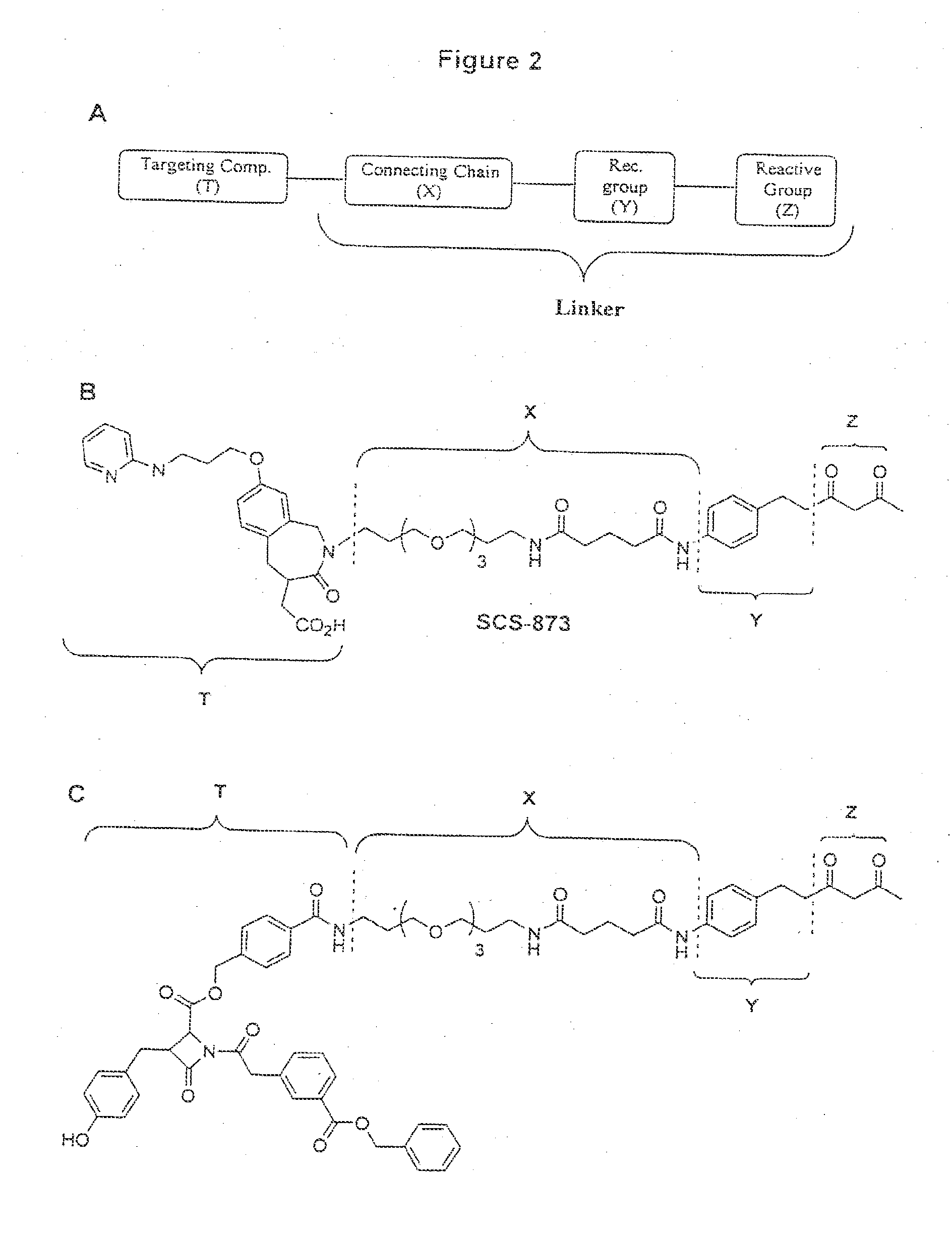
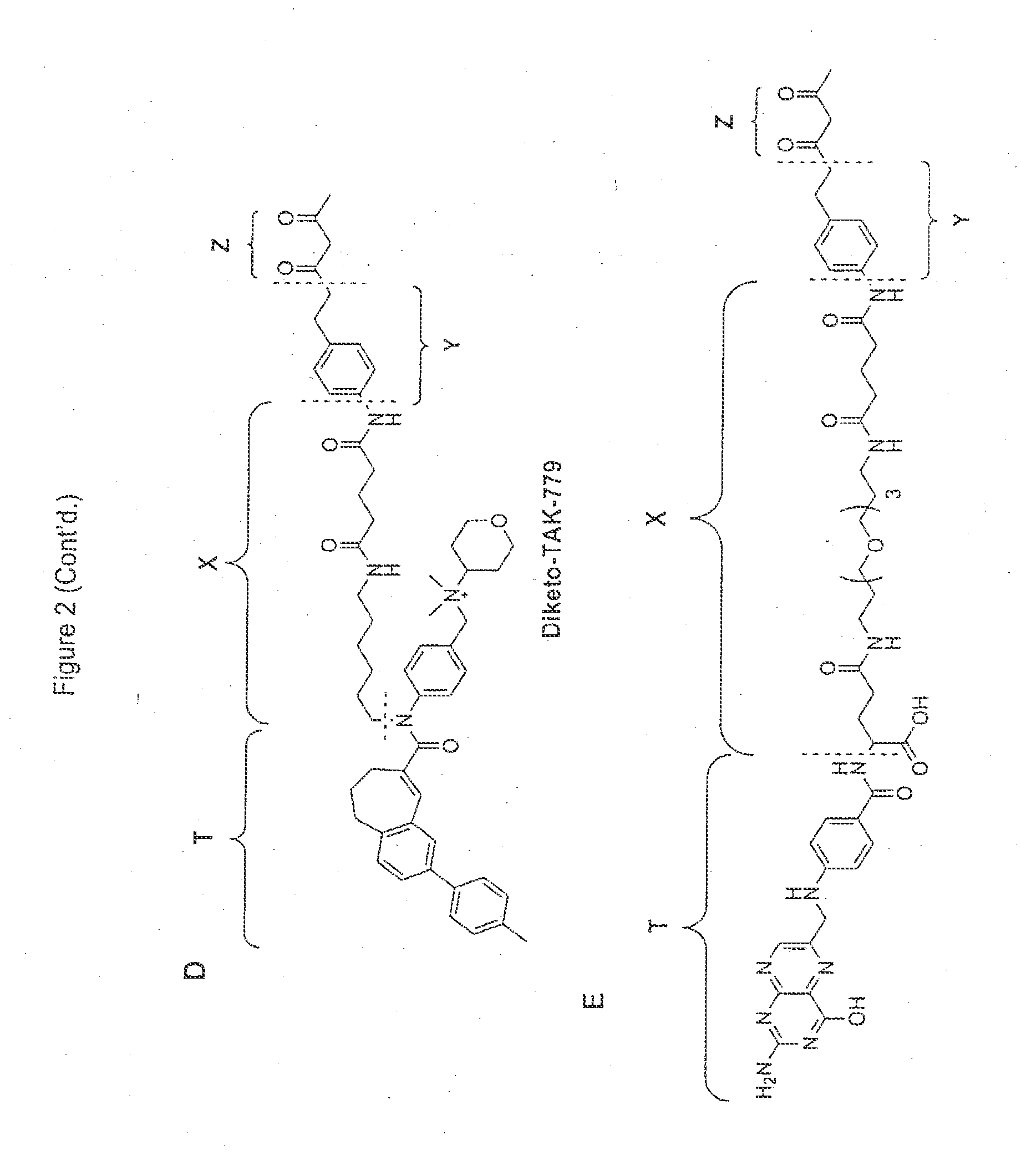
Targeting compounds
a technology of biological molecules and compounds, applied in the field of targeting compounds, can solve the problems that conventionally developed pharmaceutical drugs and biological effector molecules are often of limited use in therapy, and achieve the effects of reducing the ability of a targeting agent or biological agent to cross, reducing or inhibiting the ability of the agent to cross the cell membran
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Antibody Targeting Compound Comprising an RGD Peptidomimetic Targeting Agent Covalently Linked to the Combining Site of Aldolase Monoclonal Antibody 38C2
[0183]An integrin targeting compound was formed based on the formation of a reversible covalent bond between a diketone linker derivative of an RGD peptidomimetic and the reactive lysine of mouse mAb 38C2. Mouse mAb 38C2 is the prototype for a new class of catalytic antibodies generated by reactive immunization and mechanistically mimic natural aldolase enzymes (Barbas et al., Science 278, 2085-2092, 1997). Through a reactive lysine, these antibodies catalyze aldol and retro-aldol reactions using the enamine mechanism of natural aldolases (Wagner et al., Science 270, 1797-1800, 1995; Barbas et al., Science 278, 2085-2092, 1997; Zhong et al., Angew. Chem. Int. Ed. 38, 3738-3741, 1999). In addition to their versatility and efficacy in synthetic organic chemistry, aldolase antibodies have been used in the activation of camptothecin, do...
example 2
Antibody Targeting Compound Comprising IL-4 as Targeting Agent Covalently Linked to the Combining Site of Aldolase Monoclonal Antibody 38C2
[0188]Kaposi's sarcoma tumor cells, among other human epithelial tumor cells, express interleukin-4 (IL-4) receptors that can be targeted with a recombinant chimeric protein consisting of IL-4 and a truncated form of bacterial toxin called Pseudomonas exotoxin (Husain et al., 1999, Nat. Med. 5, 817-822). Based on these studies, an IL-4 targeting compound for targeting mAb 38C2 to Kaposi's sarcoma tumor cells is prepared. A linker with a diketone reactive group is conjugated to a lysine side chain of IL-2 using a lysine reactive moiety such as N-hydroxysuccinimide (NHS). Alternatively, a recombinant IL-4 with an added free cysteine is used for conjugation to cysteine reactive moieties such as maleimide. To reduce immunogenicity associated with the linker portion of the targeting compound, the spacer (i.e. linker connecting chain) between the diket...
example 3
Antibody Targeting Compound Comprising VEGF-R2 Binding Peptide as Targeting Agent Covalently Linked to the Combining Site of Aldolase Monoclonal Antibody 38C2
[0189]Vascular endothelial growth factor (VEGF) is a key modulator of tumor angiogenesis. Induced by hypoxia, VEGF expression is upregulated through the induction of VEGF mRNA transcription in the tumor. Following production and release by the tumor, VEGF diffuses to endothelial cells of nearby preexisting blood vessels, which display VEGF receptors (VEGFR). VEGF binds to two tyrosine kinase receptors, VEGFR-1 and VEGFR-2, which are expressed predominantly on endothelial cells. Activation of endothelial cells is associated with the binding of VEGF to VEGFR-2, whereas VEGFR-1 probably functions as a decoy receptor that regulates the local concentration of VEGF (Neufeld et al., 1999, FASEB J. 13, 9-22). Following activation, the endothelial cells proliferate, migrate directionally toward the tumor, and eventually roll up and inte...
PUM
| Property | Measurement | Unit |
|---|---|---|
| intramolecular distance | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com