Recombinant therapeutic glycine n-acyltransferase
a technology of acyltransferase and glycine, which is applied in the direction of transferases, peptide/protein ingredients, metabolic disorders, etc., can solve the problems of affecting the glycine conjugation capacity of humans, and affecting the glycine conjugation capacity. , to achieve the effect of enhancing detoxification, enhancing detoxification in mammals, and treating and/or preventing metabolic disorders
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Recombinant Bovine GLYAT
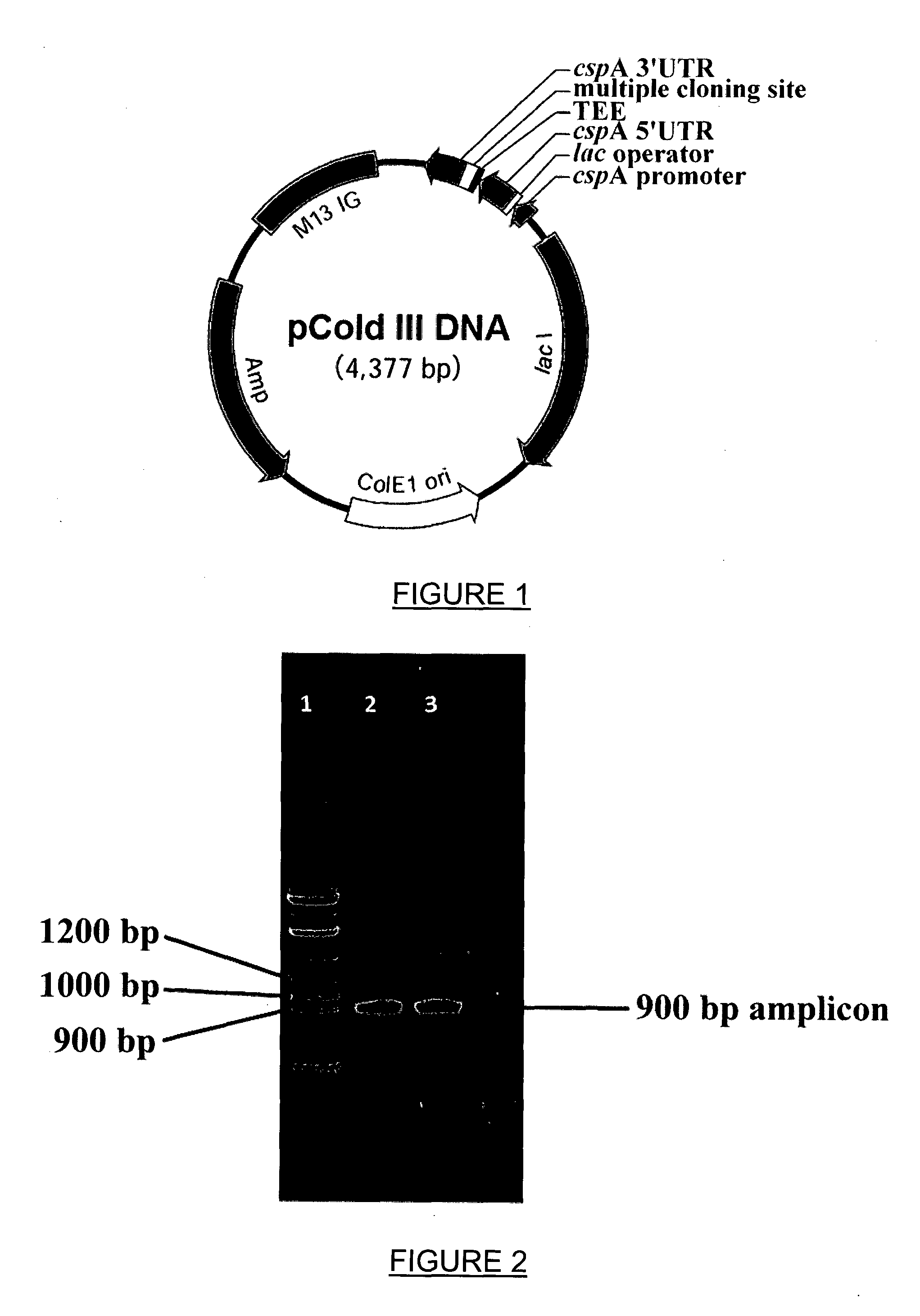
[0059]Recombinant bovine GLYAT was cloned into a set of three modified pColdIII (pColdIII-E, pColdIII-A and pColdIII-EH) expression vectors encoding C-terminal histidine tags.
[0060]In order to clone the coding sequence into the expression vectors, the sequence is amplified through polymerase chain reaction (PCR) using primers containing Ndel and Xhol restriction enzyme sites to facilitate directional cloning. The PCR reaction mixtures contained 1X Takara ExTaq buffer, 10 nmol of each dNTP, 25 pmol of each primer, approximately 50 ng of template DNA and 2 units of Takara ExTaq polymerase, in a final volume of 50 μl. Thermal cycling conditions were 94 degrees Celsius for 1 min, then 30 cycles of 94 degrees Celsius for 30 seconds, 70 degrees Celsius for 30 seconds, and 72 degrees Celsius for 1 minute, followed by a final extension at 72 degrees Celsius for 10 minutes.
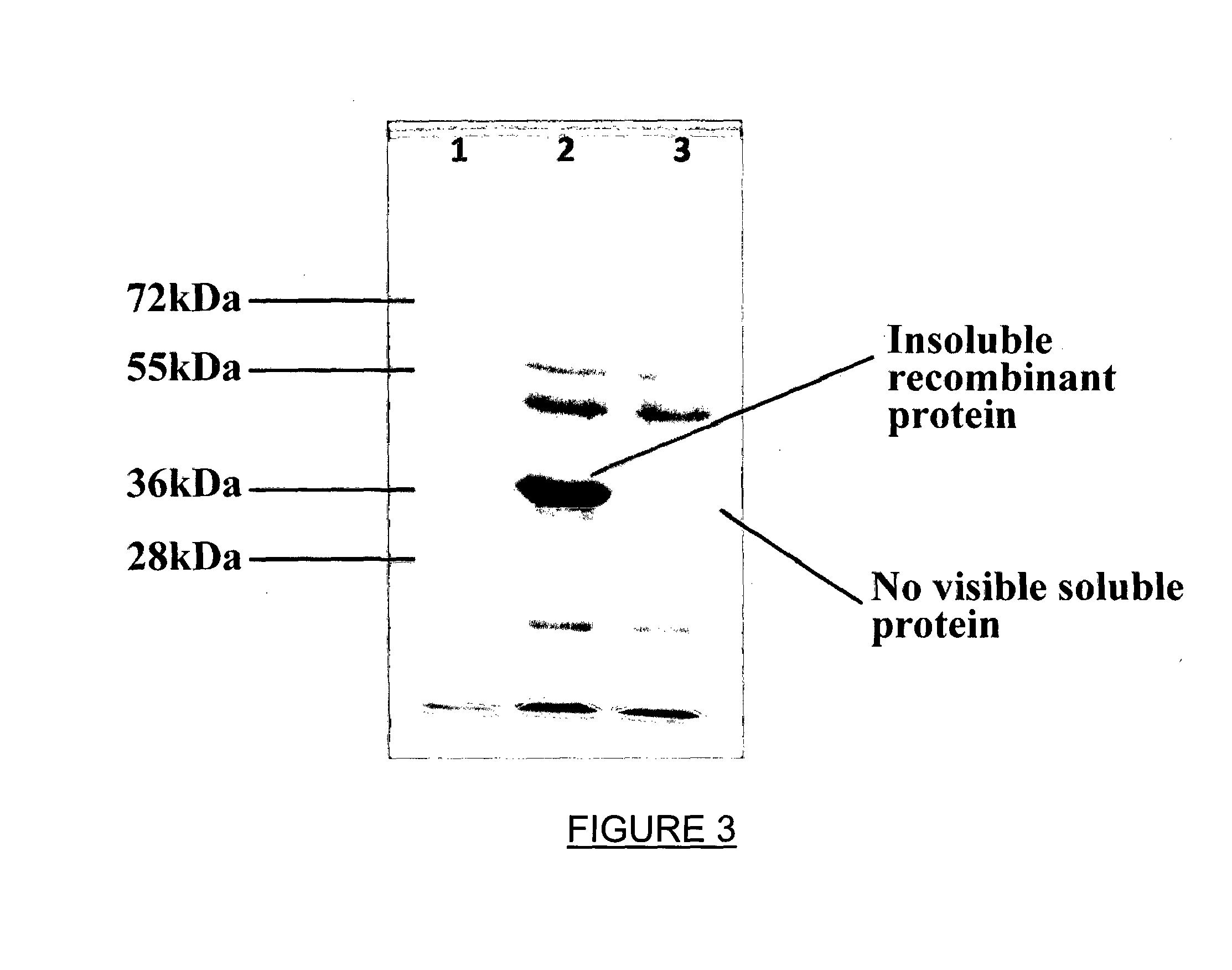
[0061]After transforming E. coli with an expression plasmid containing a recombinant GLYAT codin...
example 2
Recombinant Human GLYAT
[0068]The nucleotide sequence encoding the human GLYAT sequence was synthesised and cloned into the pET32 expression vector.
[0069]The pET32 expression vector enables the expression of human GLYAT with an N-terminal hexahistidine tag and an N-terminal Trx-tag, which respectively facilitates the purification and correct folding of the enzyme.
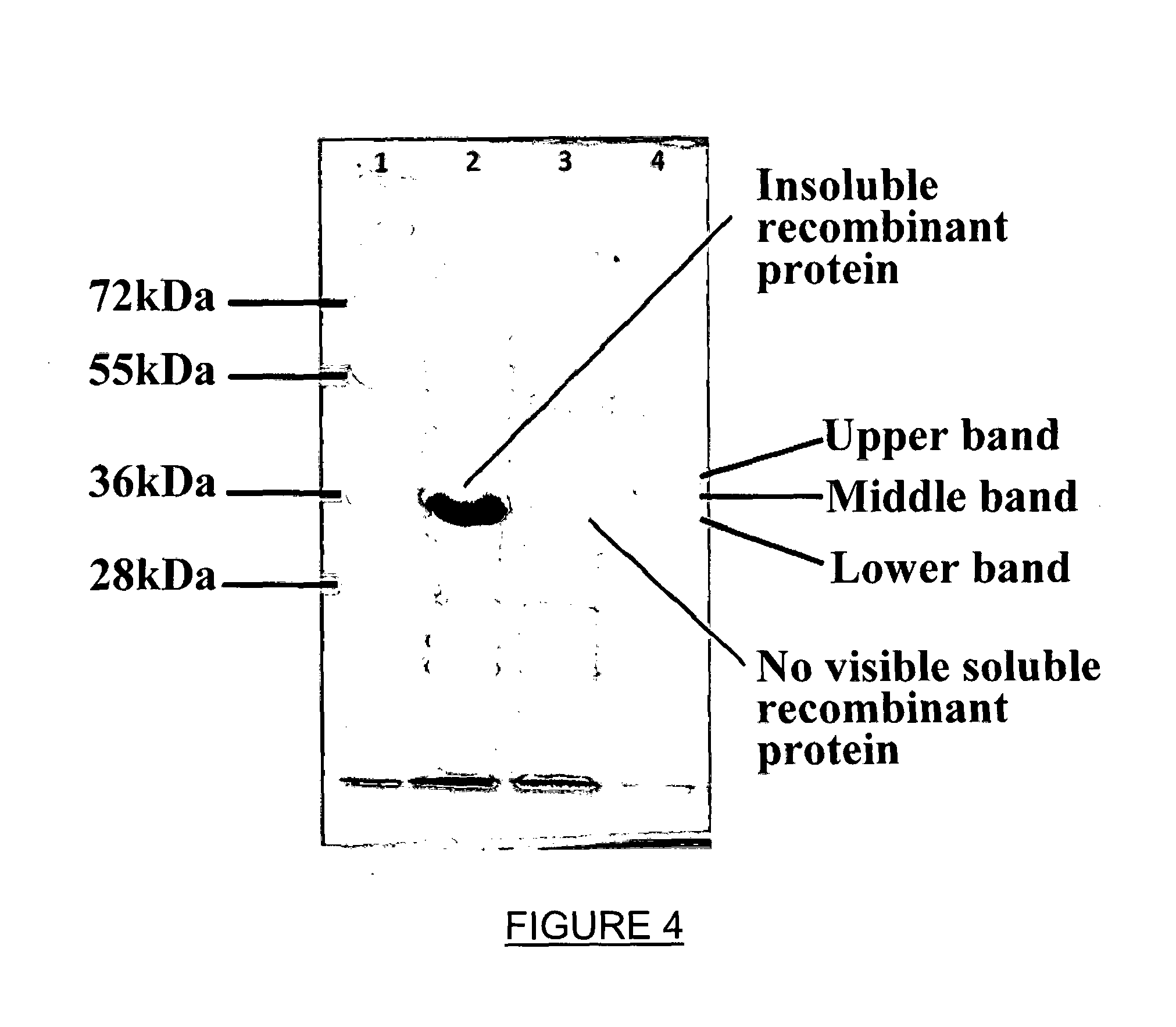
[0070]The expression vector encoding human GLYAT was transformed into Origami expression cells. The cells were also transformed with the pGro7 vector from Takara, which resulted in co-expression of the GroES and GroEL chaperone proteins. Chaperone proteins aid in the correct folding of proteins and increase the yield of soluble recombinant enzymes.
[0071]The Origami cells containing the plasmids for expression of recombinant human GLYAT and the chaperone proteins were grown in liquid culture. It was found that the optimal expression of soluble GLYAT occurs in the absence of IPTG (Isopropyl (β-D-1-thiogalactopyranoside), thus ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| body weight | aaaaa | aaaaa |
| body weight | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com