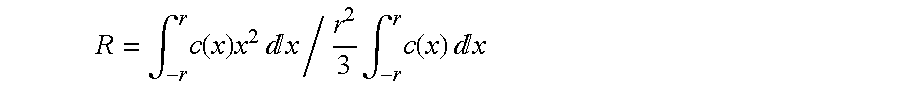Catalyst comprising palladium and silver, and its application for selective hydrogenation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of a Catalyst C1 According to the Invention
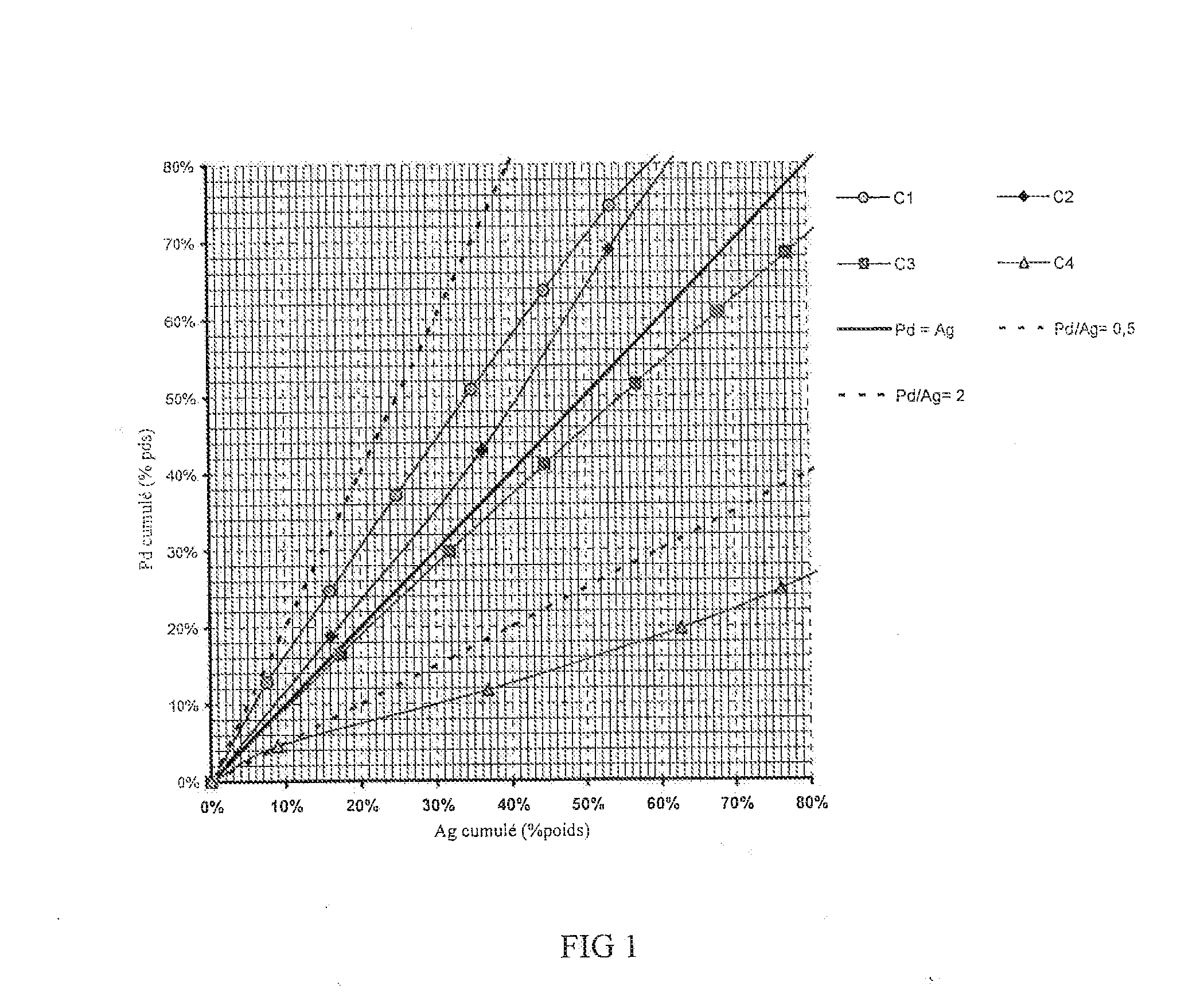
[0143]This example shows the preparation of a catalyst according to the invention comprising impregnation of the support in two stages according to the process of the invention, that is, by using a colloidal solution for the Pd. The catalyst obtained comprises a fine crust of palladium and silver having a proximity ratio PR on a scale of 0.5 to 2.
[0144]A colloidal suspension of Pd oxide is prepared under agitation at 25° C. by diluting 1.8 g of a solution of palladium nitrate Pd(NO3)2 containing 8.5 wt. % of palladium Pd with approximately 45 ml demineralised water, then adding approximately 10 ml of sodium hydroxide solution to give a pH of 2.4. The suspension is then diluted with demineralised water to a volume which corresponds to the pore volume of the alumina support. This solution is then impregnated onto 80 grams of an alumina having a specific surface area of 71 m2 / g, moulded into the form of beads. A maturation step for...
example 2
Preparation of a Catalyst C2 According to the Invention
[0148]This example is identical to Example 1 with the exception of the palladium concentration, which is half as high as in Example 1. This gives a catalyst with a thinner crust and a better proximity factor PR.
[0149]A colloidal suspension of Pd oxide is prepared under agitation at 25° C. by diluting 0.95 g of a solution of palladium nitrate Pd(NO3)2 containing 8.5 wt. % of palladium Pd with approximately 45 ml demineralised water, then adding approximately 10 ml of sodium hydroxide solution to give a pH of 2.4. The suspension is then diluted with demineralised water to a volume which corresponds to the pore volume of the alumina support. This solution is then impregnated onto 80 grams of an alumina having a specific surface area of 71 m2 / g, moulded into the form of beads. A maturation step for the impregnated support is carried out before drying in air in a confined moist medium, for a period of 20 h. The solid obtained is drie...
example 3
Preparation of a Catalyst C3 not Conforming to the Invention
[0153]This example shows the preparation of a catalyst not conforming to the invention comprising impregnation of the support in two stages according to conventional impregnation methods (that is, without using a colloidal solution for the Pd). The catalyst obtained comprises a relatively thick crust, but having a proximity ratio PR on a scale of 0.5 to 2.
[0154]A solution of palladium nitrate is prepared under agitation at 25° C. by diluting 2.7 g of a solution of palladium nitrate Pd(NO3)2 containing 8.5 wt. % of palladium Pd with approximately 50 ml demineralised water. This solution is then impregnated onto 80 g of an alumina having a specific surface area of 71 m2 / g, moulded into the form of beads. The solid obtained is dried in air for 2 h at 120° C. The catalyst is then calcined in a stream of air at 450° C. for 2 h.
[0155]The solid obtained is immersed in 500 ml of a 2.3 g / l solution of formic acid. The solution is ag...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com