Stability of gas atomized reactive powders through multiple step in-situ passivation
a technology of reactive powders and gas atomization, which is applied in the direction of metal-working apparatuses, explosives, transportation and packaging, etc., can solve the problems of pyrophoric powders, achieve the effects of improving the thermal ignition temperature and spark ignition resistance of atomized particles, improving ignition stability, and being more resistant to ignition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
[0022]The following Example for making magnesium powder is offered to further illustrate but not limit the present invention:
Experimental Procedure
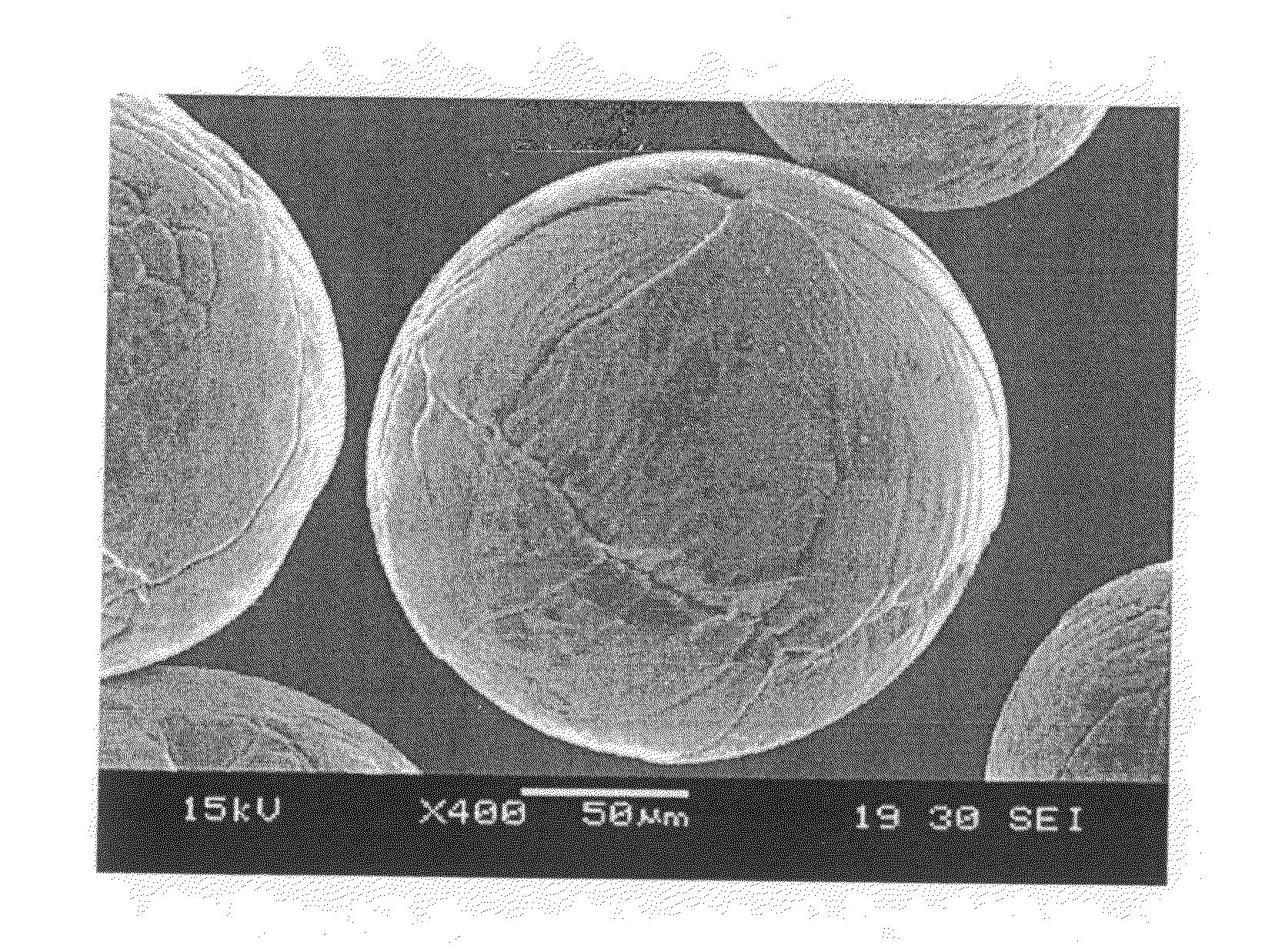
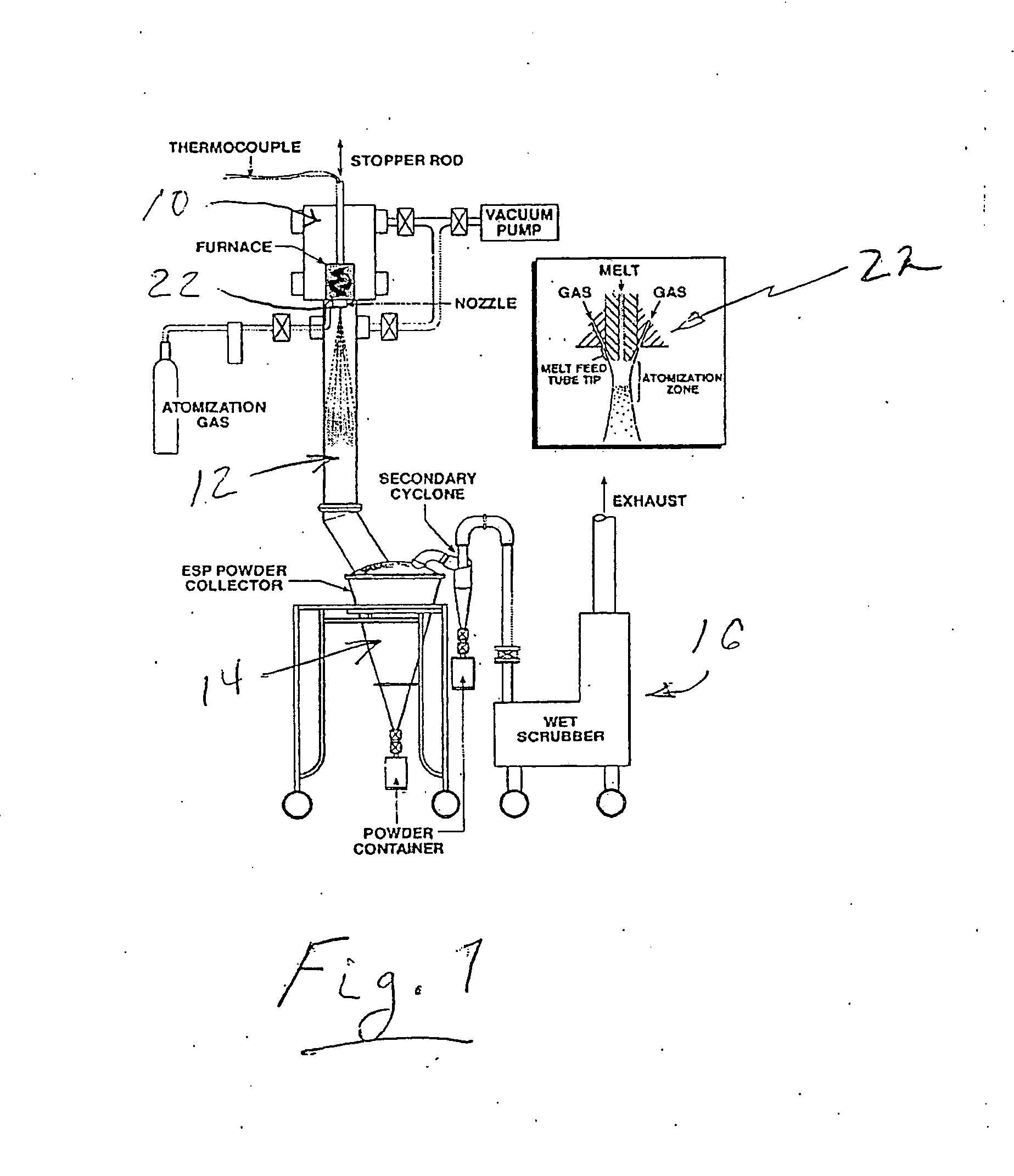
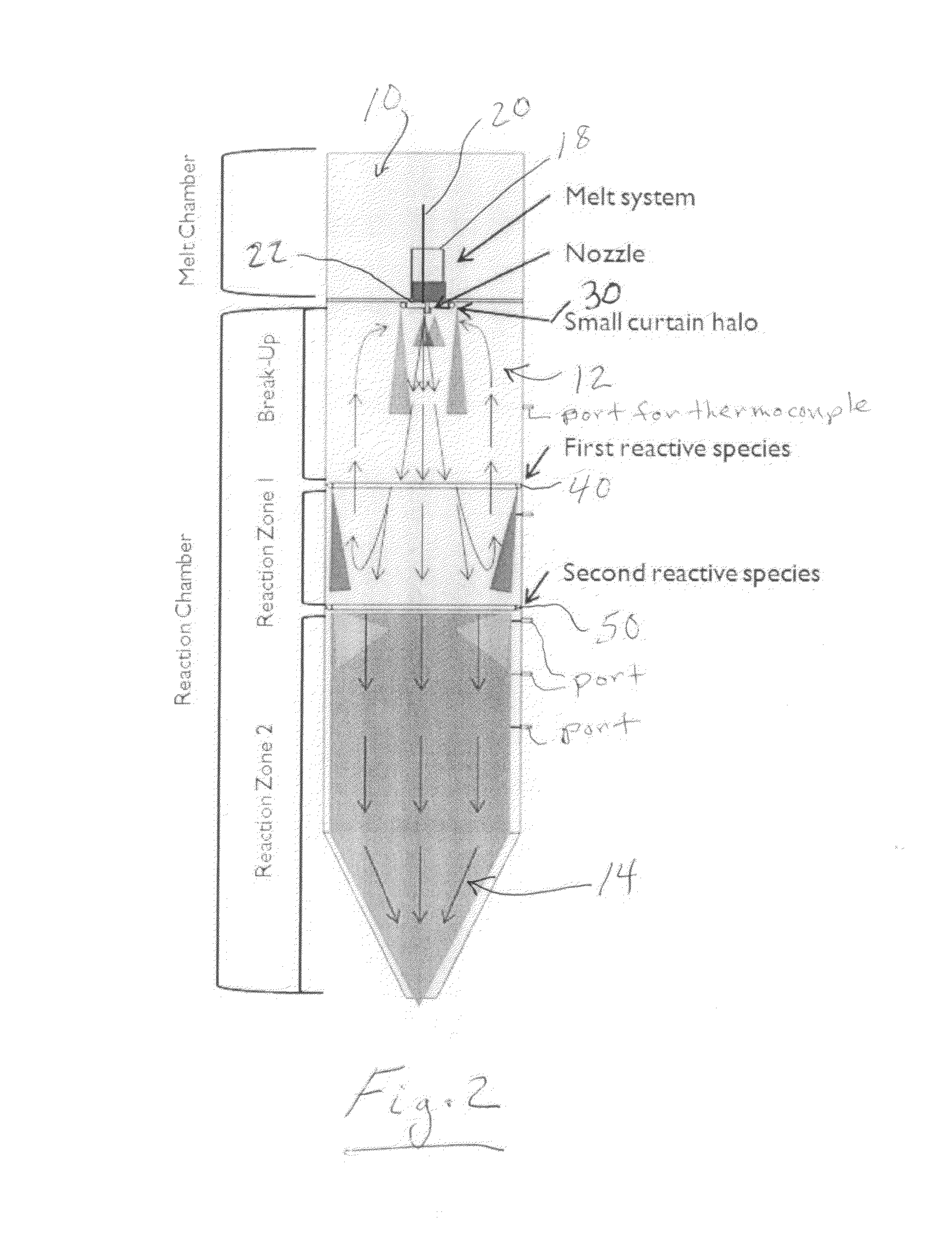
[0023]A modified high pressure gas atomization system (HPGA) located at the Ames laboratory, Ames, Iowa, was used for conducting low pressure gas atomization (LPGA) of magnesium to produce passivated magnesium powders in accordance with an illustrative embodiment of the present invention. The gas atomization system is generally as described in U.S. Pat. Nos. 5,372,629; 5,589,199; and 5,811,187, which are incorporated herein by reference and is shown schematically in FIG. 1. The basic gas atomization system includes a melting chamber 10, a drop tube (spray chamber) 12 beneath the melting chamber and which was 2 feet in diameter and approximately 9 feet tall, and a powder collection chamber 14 along with an exhaust cleaning system 16. The melting chamber 10 on top of the spray chamber 12 contained the melt system. The melt system included a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com