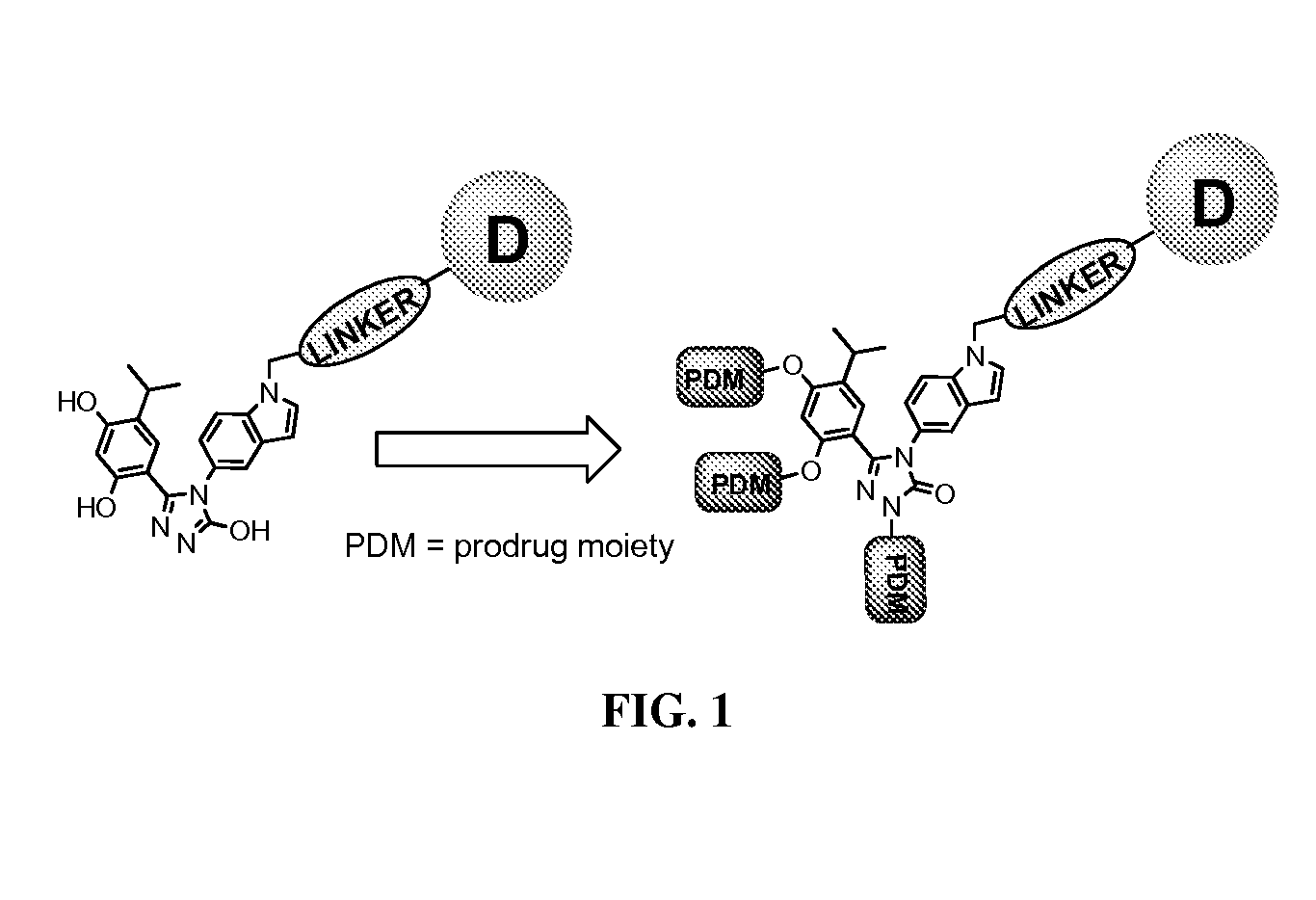
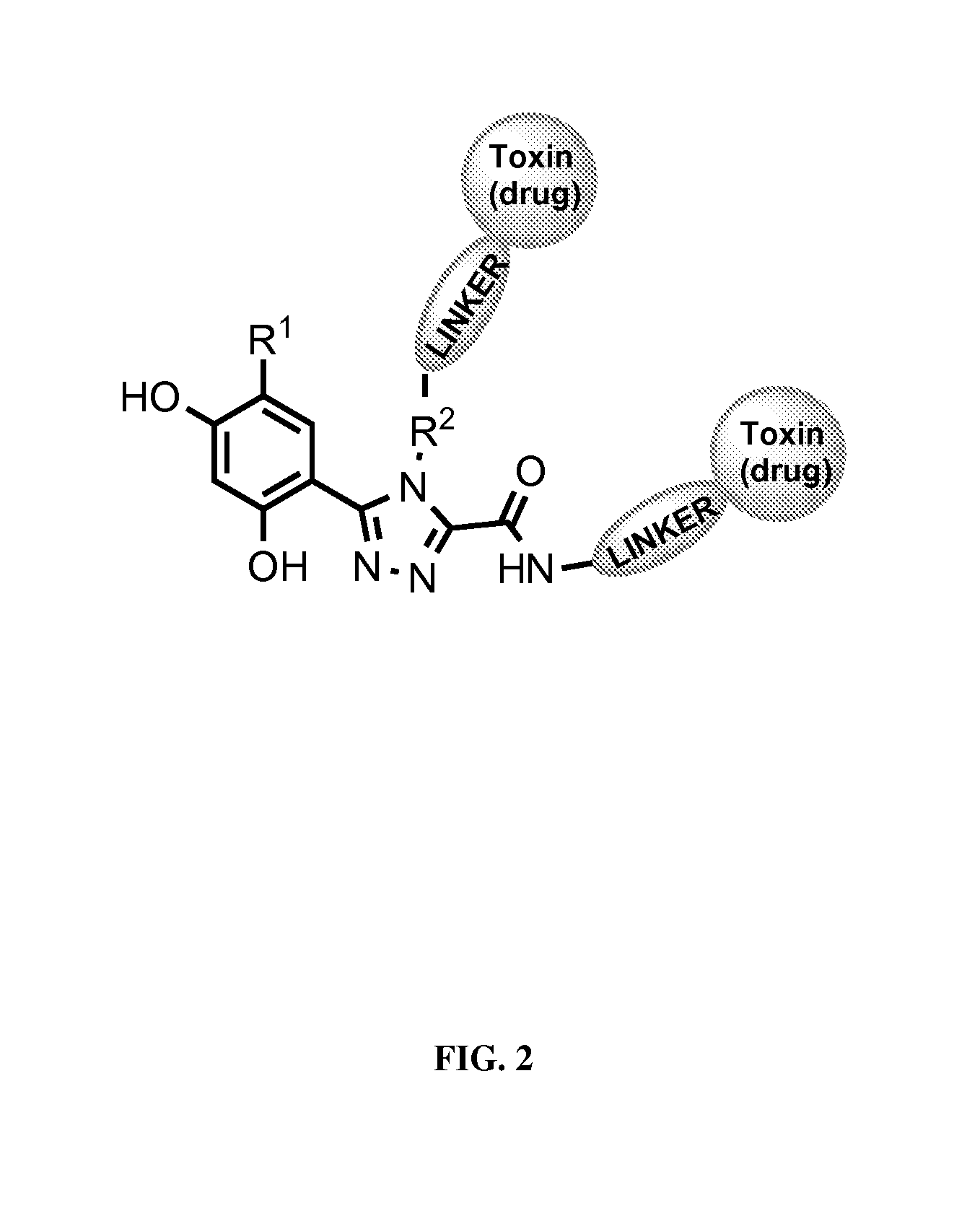
Targeted therapeutics
a targeted, therapeutic technology, applied in the direction of biocide, drug composition, instruments, etc., can solve the problems of unsatisfactory current therapeutics and therapies, limited applicability and/or effectiveness of chemotherapy, and limited side effects of other therapies and diagnostics employing potentially toxic moieties, so as to facilitate an additive or synergistic
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0355]The following examples, which are briefly summarized and then discussed in turn below, are offered by way of illustration and not by way of limitation.
[0356]Example 1 presents the synthesis of exemplary SDC-TRAPs.
[0357]Example 2 presents the targeted delivery of exemplary SDC-TRAPs.
[0358]Example 3 presents an exemplary assay for selecting binding moieties.
[0359]Example 4 presents the cytotoxicity of exemplary SDC-TRAPs.
[0360]Example 5 presents the stability of exemplary SDC-TRAPs in plasma.
[0361]Example 6 presents a detailed schematic for the synthesis of an exemplary SDC-TRAP.
[0362]Example 7 presents results of tests using the SDC-TRAP of Example 6.
[0363]Example 8 presents the synthesis and testing of a lenalidomide-based SDC-TRAP.
[0364]Examples 9 and 10 present examples of IC50 value determinations.
[0365]Example 11 presents an exemplary Hsp90α binding assay.
[0366]Example 12 presents an exemplary HER2 degradation assay.
[0367]Example 13 presents an exemplary cytotoxicity assay...
example 1
[0375]SDC-TRAPs of an exemplary embodiment may be prepared in following manner:
[0376]The synthesis of Compound 1 and Compound 3 are discussed in WO 2007 / 139968 and WO 2004 / 012661, respectively.
[0377]Synthesis of Compound 2 (STEP-1): To a solution of 1.0 g (2.48 mmols) of compound 1 in 60 mL of 1:1:1-Methanol:Tetrahydrofuran:Acetic acid was added 75 mg of 10% Palladium on charcoal (wet Degussa type) and the contents of the flask was deoxygenated by vacuum and hydrogen purge. It was then pressurized to 60 Psi with hydrogen and stirred for 5 h at room temperature. The flask was then thoroughly flushed with argon and filtered the solids through a short pad of celite. Evaporation and recrystallization of the crude product afforded 900 mg (88%) of the compound 2 in pure form as off white solid. ESMS calculated for C23H28N4O3: 408.22. Found: 409.1 (M+).
[0378]Synthesis of the Conjugate 1: To a stirred solution of 0.1 g (0.245 mmols) of Compound 2 in 5 mL of anhydrous N,N-Dimethylformamide w...
example 2
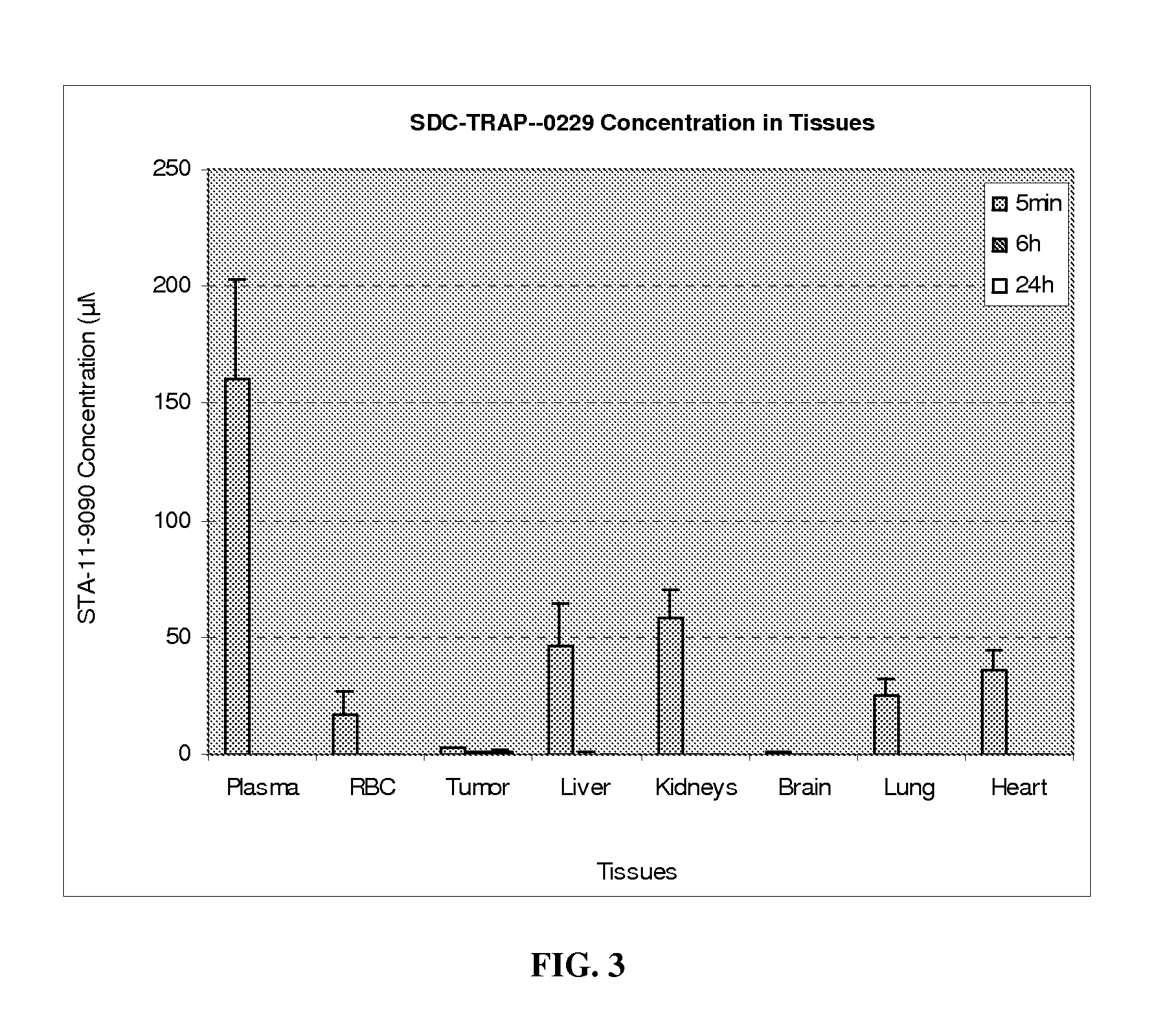
[0450]The ability of Hsp90-targeting moieties to penetrate solid tumors and exhibit rapid clearance from normal tissues for reduced toxicity is illustrated in the following tissue distribution study with a compound, ganetespib, which may be used as an Hsp90 binding moiety.
[0451]Tissue Distribution of ganetespib in Female CD-1 nu / nu Mice Bearing RERF Human NSCLC Xenografts
[0452]Objectives:
[0453]To confirm the distribution of ganetespib in blood, livers, kidneys, brains, hearts, lungs and tumors after IV administration of ganetespib to female CD-1 nu / nu mice bearing RERF human NSCLC xenografts, and to examine metabolic profiles of ganetespib in plasma, red blood cells, and above tissues.
[0454]Study Outline:
[0455]Test Articles: ganetespib
Animals: female CD-1 nu / nu mice bearing RERF human NSCLC xenografts (N=3 / group)
Route: IV
[0456]Dosage: 50 mg / kg
Dose level: 10 mL / kg
Formulation: 10% DMSO, 18% Cremophor RH40, 3.6% dextrose solution (DRD)
Bleeding time points: 5 min, 6, 24 hr
Collected tiss...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com