Process for the production of chlorinated propenes
a chlorinated propene and propene technology, applied in the field of chlorinated propene production processes, can solve the problems of prohibitively high cost, limited commercial availability of many chlorinated propenes, and prohibitively expensive to be economically produced, etc., to achieve enhanced process selectivity, low cost, and fewer steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
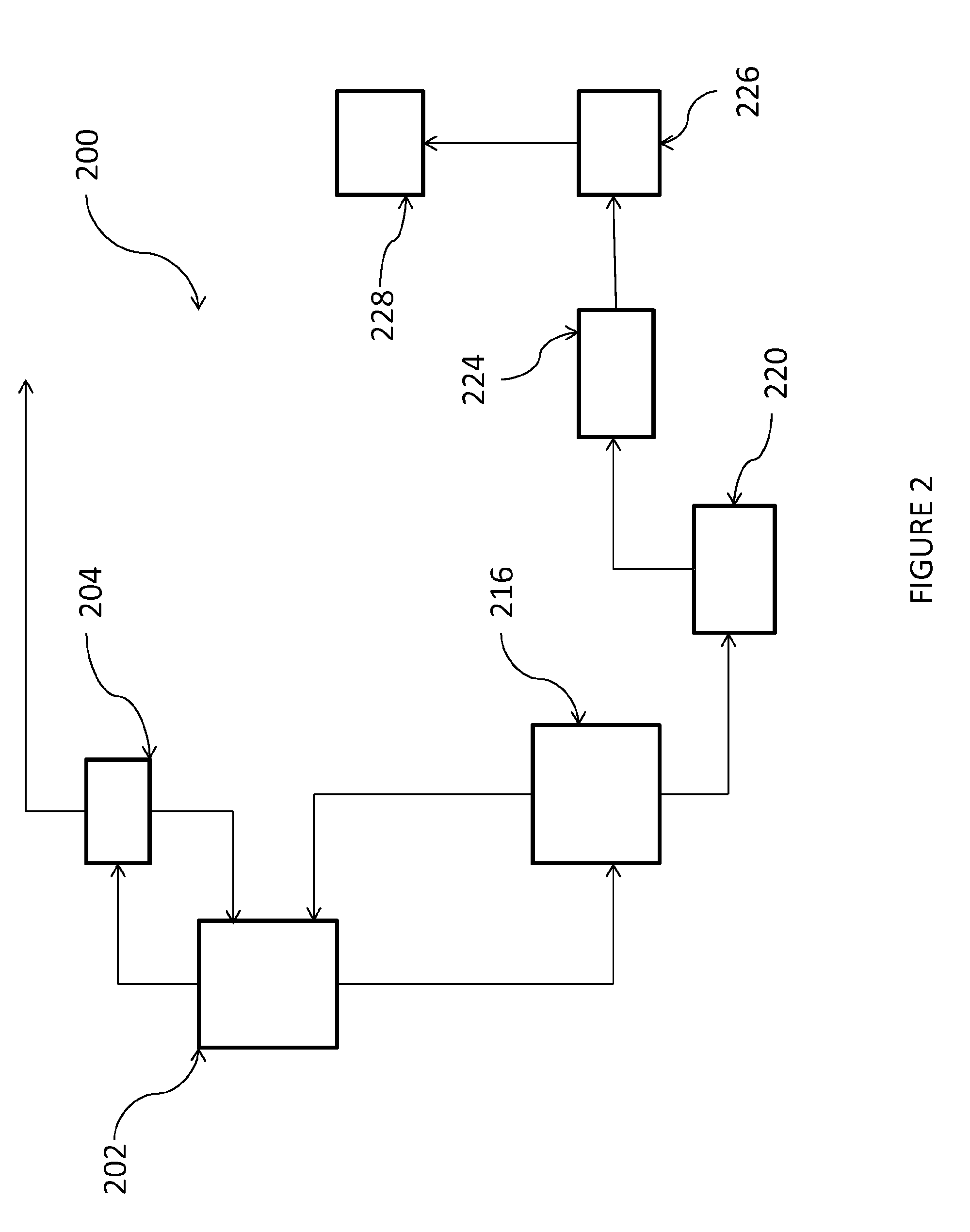
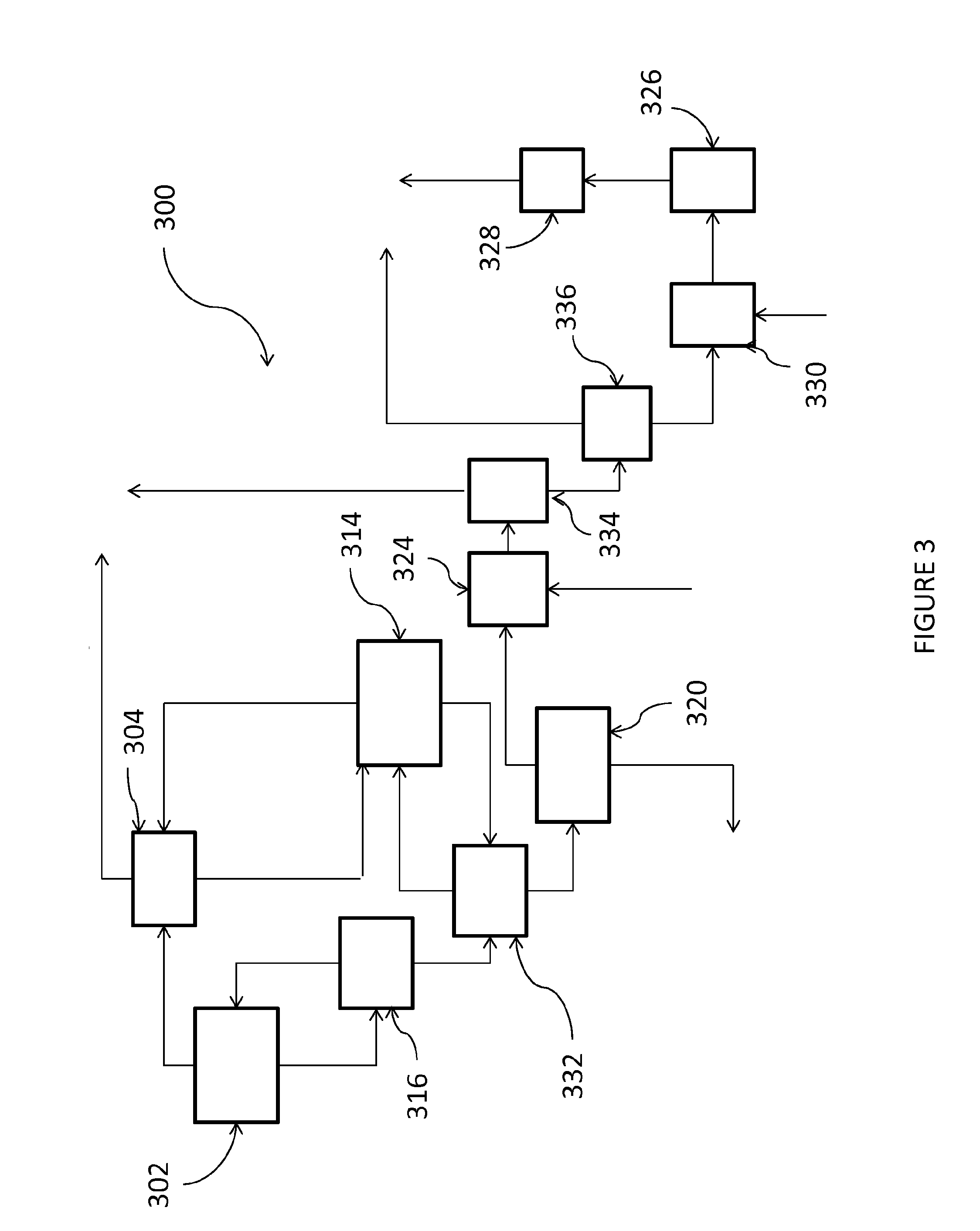
Image
Examples
example i
[0080]In this example, liquid SO2Cl2 and PDC (1,2-dichloropropane) are mixed in a 100 ml flask heated in a water bath to maintain temperature 55° C. to 60° C. A reflux column is placed to return unreacted sulfuryl chloride and PDC, that are stripped by SO2 and HCl byproducts, to the reaction flask.
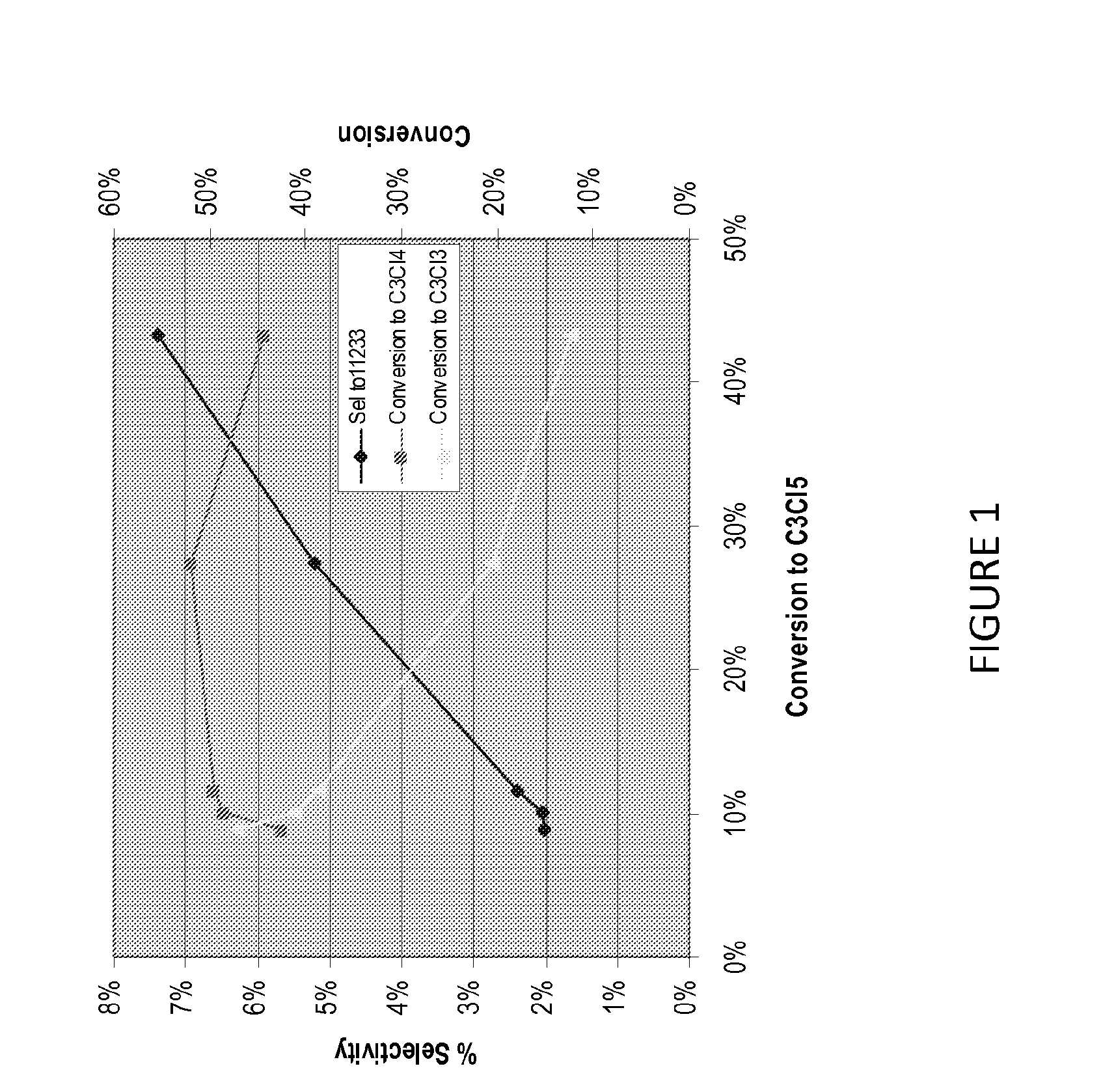
[0081]Table 1 shows the product distribution, as determined by GC / MS, at various SO2Cl2 and / or AIBN initiator concentrations at near complete PDC conversions. As shown by both Table 1 and FIG. 1, this process, using high excess SO2Cl2 at 45% total conversion to pentachloropropane isomers, provides less than 8% molar selectivity to the less desirable pentachloropropane, 1,1,2,3,3-pentachloropropane (11233).
TABLE 1SO2Cl2 / PDC33556AIBN / PDC02123PDC98.5%100.0%100.0%100.0%100.0%conversionSelectivity112233.3%3.7%5.0%11.8%19.4%112332.0%2.0%2.4%5.2%7.4%111221.3%1.7%2.5%6.3%10.7%111232.3%2.6%1.7%4.1%5.8% 112213.2%17.8%19.4%21.2%23.9% 112315.6%15.6%14.8%10.8%8.9% 122310.1%11.8%12.3%12.9%9.7% 11123.6%3...
example ii
[0082]In this example, PDC (10 mL) is mixed in a 100 ml pressure reactor with 2,2′-azobis(2,4-dimethyl valeronitrile) (100 mg), dimethyl 2,2′-azobis(2-methylpropionate) (100 mg) or 1,1′-azobis(cyclohexane-1-carbonitrile) (100 mg) in carbon tetrachloride solvent (37.2 mL). The reactor is heated using a heating mantle to a temperature of ˜70° C. A flow of gaseous Cl2 in N2 (30% v / v at STP) is passed through the mixture at a rate of about 100 sccm for the rest of the synthesis. A reactor pressure of ˜130 psig is maintained during the experiment. 1H NMR spectroscopy is used to determine the product composition.
[0083]Table 2 shows the chlorinated product distribution after about 200 and 300 minutes of chlorine passing through solution. As also shown in Table 2 or FIG. 1, less than 8% molar selectivity to the less desirable byproduct 1,1,2,3,3-pentachloropropane (11233) is observed at all conversions.
TABLE 22,2′-azobis(2,4-dimethyl 2,2′-1,1′-dimethylazobis(2-azobis(cyclohexane-valeronitri...
example iii
[0084]In this example, a 100 ml pressure reactor is charged with carbon tetrachloride (45 mL) and gaseous Cl2 in N2 (30% v / v at STP) is passed through the mixture while the pressure is held at about 150 psig until saturation is reached. The reactor is heated using a heating mantle to a temperature of ˜70° C. and while the pressure is maintained at about 150 psig. Then, a solution containing PDC (10 mL) and free radical initiator dimethyl 2,2′-azobis(2-methylpropionate) (100 mg) is added. 1H NMR spectroscopy is used to determine the product composition.
[0085]Table 3 shows the chlorinated C3 product distribution at various PDC conversions. As also shown in FIG. 1, less than 8% molar selectivity to the less desirable byproduct 1,1,2,3,3-pentachloropropane (11233) is observed at all conversions.
TABLE 3Sample 1Sample 2Time1732PDC98.999.6ConversionSelectivity112232.6%3.7%112331.1%1.6%111222.2%3.3%111230.7%1.0% 112220.1%23.0% 112311.5%12.1% 122310.9%12.3% 11123.5%3.6% 1128.5%6.2% 12329.9%2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com