Methods of making patterned structures of materials, patterned structures of materials, and methods of using same
a technology of patterned structures and materials, applied in the field of patterning materials, can solve the problems of unattractive lithography community, significant swelling, intermixing or damage to the film of polymer a, and the limitation of only one self-assembled block copolymer domain size and pitch
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0068]In the following example, two different block copolymers were spin-coated adjacent to each other in the same layer to demonstrate the CASP technique. Hydrofluoroether (HFE) (Novec™) solvents were obtained from 3M Co. and used as received. “OSCoR” photoresist was supplied by Orthogonal Inc. Triphenyl sulfonium triflate (TPST) photoacid generator was obtained from Aldrich and used as received. Tetramethoxymethylglycouril (TMMGU, “Powderlink 1174”) was donated by Cytec Industries. All other solvents were obtained from Aldrich and used as received. PαMS-b-PHOST block copolymers (Mn=53,000 g / mol, fPαMS˜34% (BCP1) and Mn=23,000 g / mol, fPαMS˜30% (BCP2) were synthesized as reported previously. Poly(styrene) with an —OH end group (PS—OH, Mn=10,000 g / mol, PDI=1.07) was obtained from Polymer Source (Dorval, Canada) and used as received. Single-polished Silicon wafers containing a ˜2 nm native oxide layer were obtained from WRS (Spring City, Pa.).
example 2
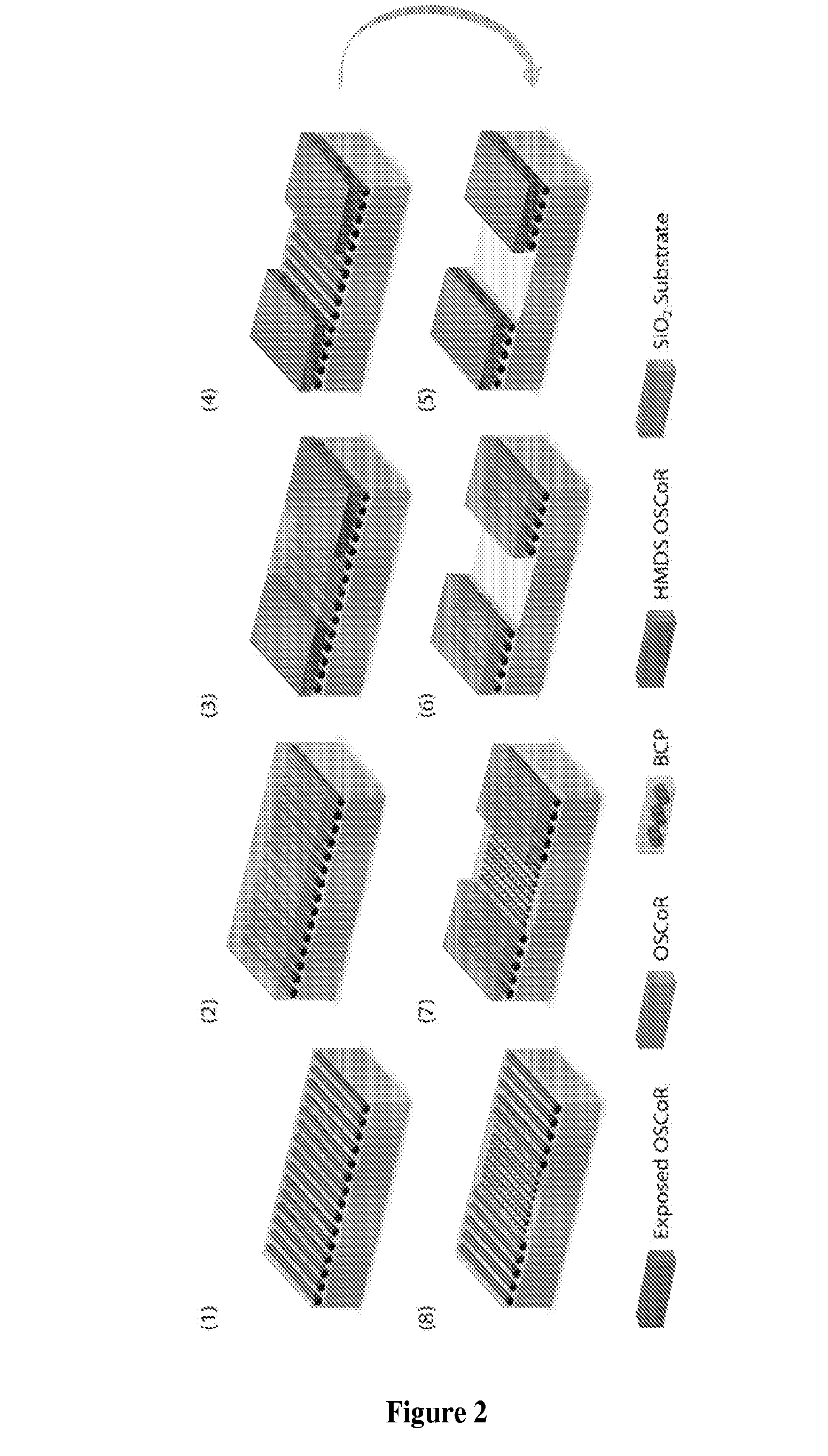
[0078]In this example the non-interaction between the block copolymer film and hydrofluoroether (HFE) solvents was investigated for orthogonal processing
[0079]Materials. Hydrofluoroether solvents (HFEs, Novec™ Engineered Fluids) were obtained from 3M Corporation and used as received. Organic SemiConductor Resist (OSCoR) was supplied by Orthogonal Inc. Triphenyl sulfonium triflate (TPST, photoacid generator) was obtained from Sigma-Aldrich and used as received. Tetramethoxymethylglycouril (TMMGU, “Powderlink 1174”) was donated by Cytec Industries. All other solvents were obtained from Sigma-Aldrich and used as received. PαMS-b-PHOST block copolymers (Mn=53,000 g / mol, fPαMS˜34% (BCP1) and Mn=23,000 g / mol, fPαMS˜30% (BCP2) were synthesized according to known literature procedures. Hydroxyl-terminated polystyrene (PS—OH) was obtained from Polymer Source and used as received. Single-polished Silicon wafers with native oxide layer were obtained from WRS Materials.
[0080]Block Copolymer Fi...
example 3
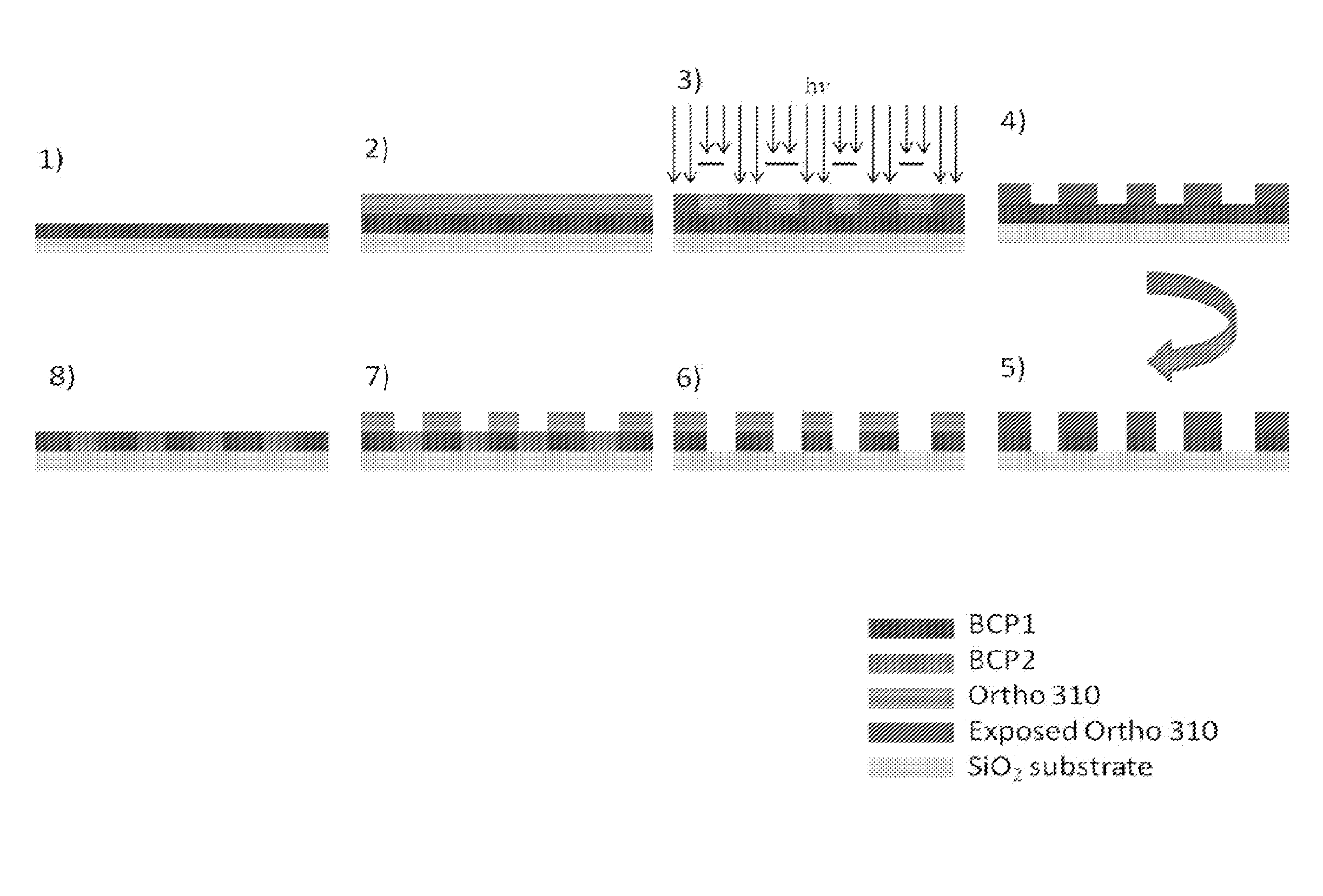
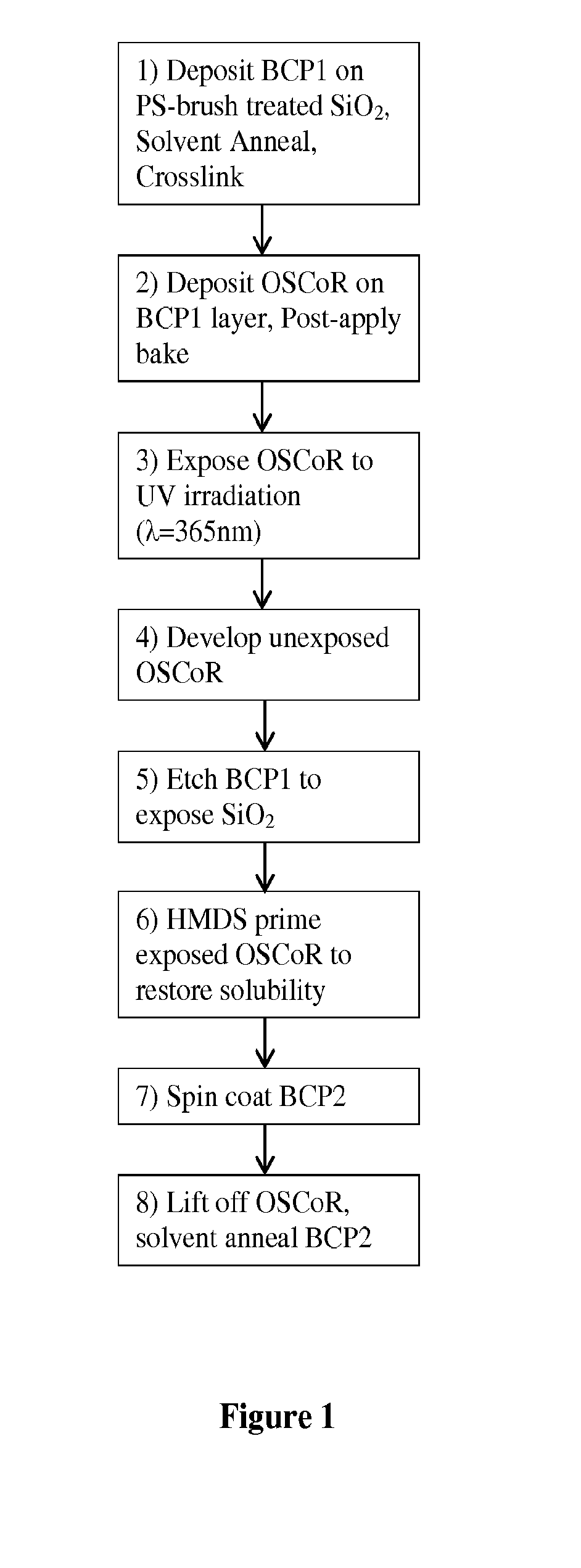
[0089]The following is an example of a method of the present invention.
[0090]Materials. Hydrofluoroether (HFE) (Novec™) solvents were obtained from 3M Corporation and used as received. Poly(1H,1H,2H,2H-perfluorodecyl methacrylate-ran-tert-butyl methacrylate) (P(FDMA-ran-TBMA), “Ortho 310”) photoresist was supplied by Orthogonal Inc. Triphenyl sulfonium triflate (TPST) photoacid generator was obtained from Aldrich and used as received. Tetramethoxymethylglycouril (TMMGU, “Powderlink 1174”) was donated by Cytec Industries. All other solvents were obtained from Aldrich and used as received. PαMS-b-PHOST block copolymers (Mn=53,000 g / mol, fαMS˜34% (BCP1) and Mn=23,000 g / mol, fPαMS˜30% (BCP2) were synthesized according to known literature procedures. Poly(styrene) with an ˜OH end group (PS—OH) was obtained from Polymer Source (Dorval, CA) and used as received. Single-polished Silicon wafers containing a ˜2 nm native oxide layer were obtained from WRS materials (Sprin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thick | aaaaa | aaaaa |
| thick | aaaaa | aaaaa |
| area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com