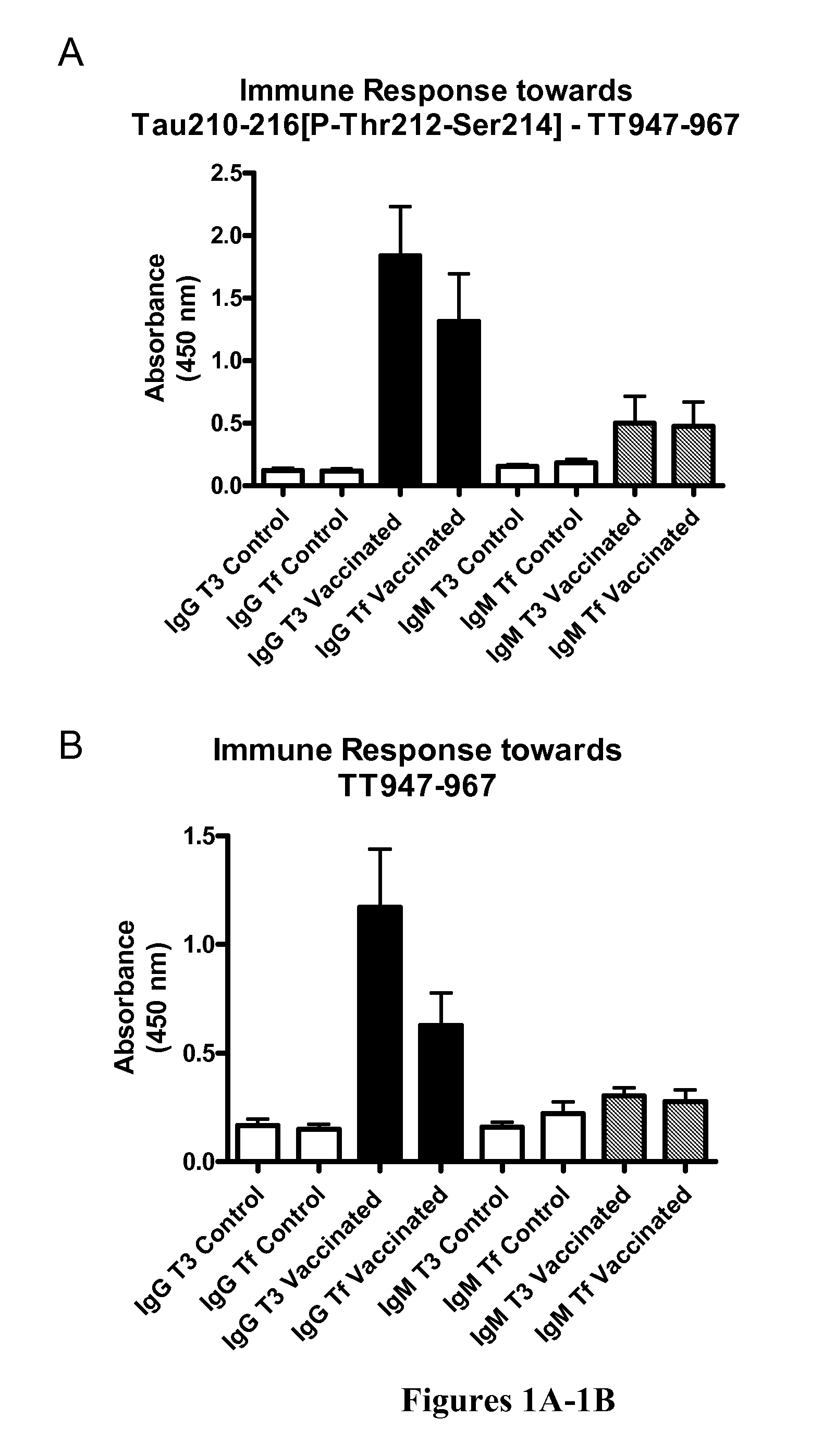
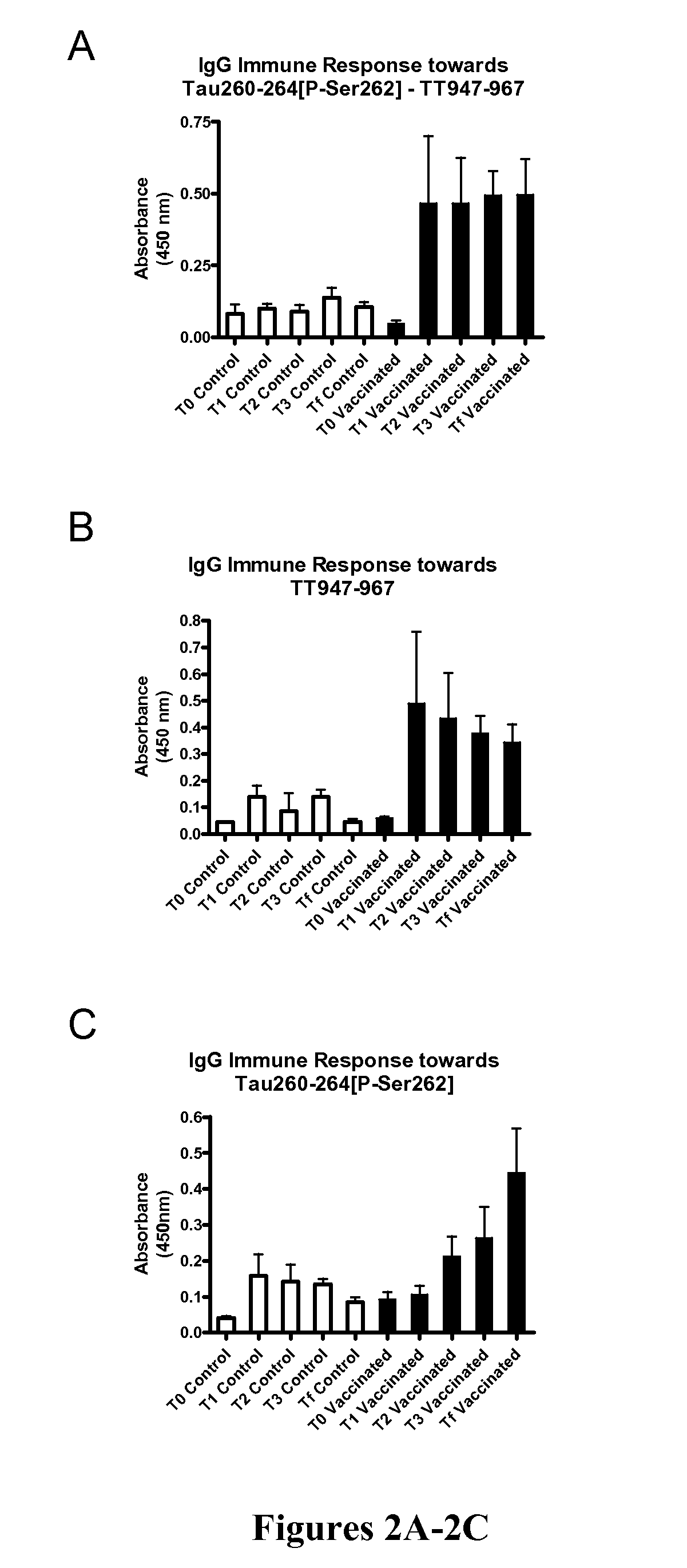
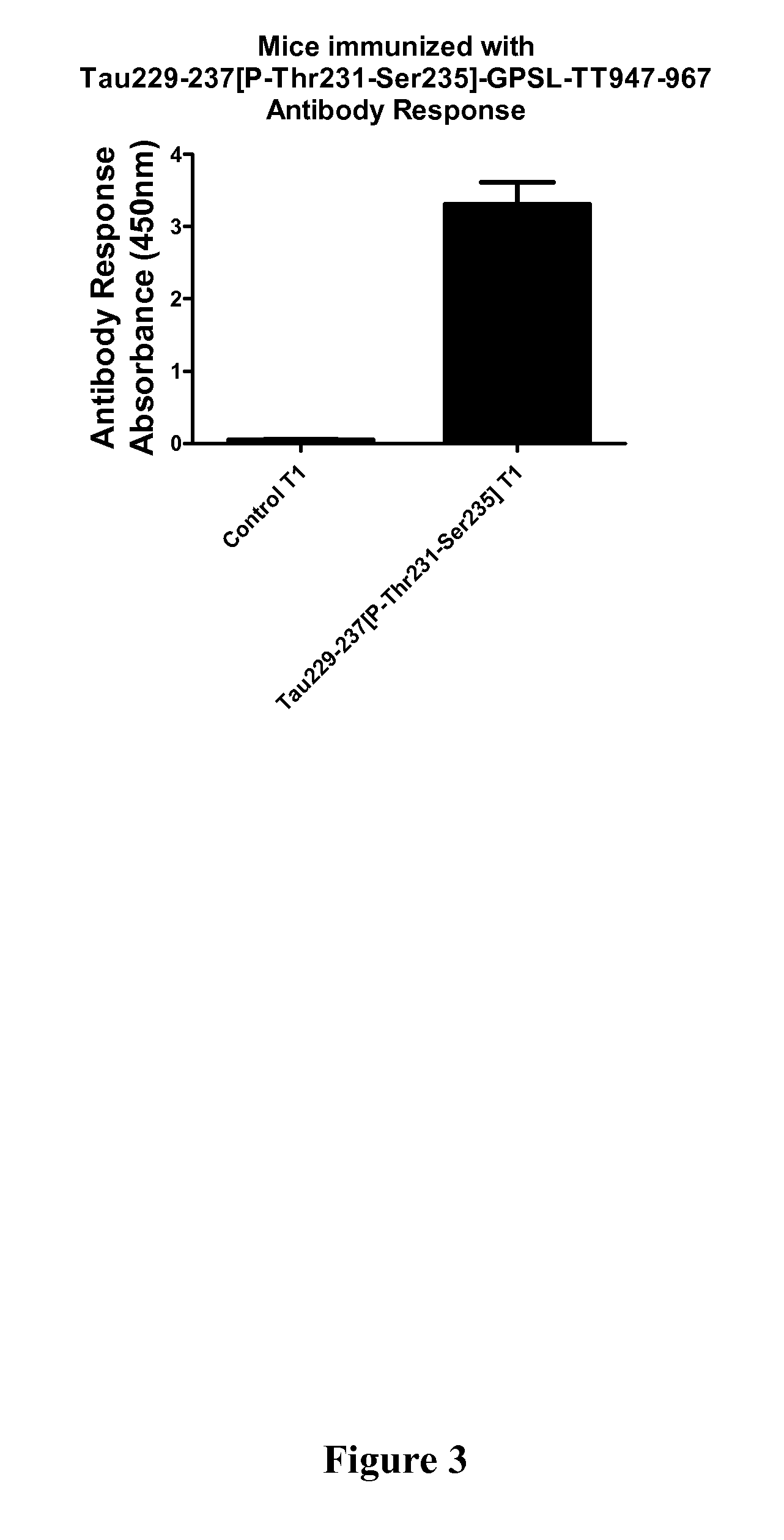
Immunological Targeting of Pathological Tau Proteins
a technology of pathological tau and immunological targeting, which is applied in the field of immunological targeting of pathological tau proteins, can solve the problems of complex cognitive assessment and sensory motor abnormalities, and achieve the effects of promoting the clearance of aggregates, slowing the progression of tau-pathology related behavioral phenotypes, and high target specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Peptides
[0103]The peptide immunogens were synthesized at the Keck facility (Yale University), by the solid-phase technique on a p-methyl-benzhydrylamine resin, using a Biosearch SAM 2 synthesizer (Biosearch, Inc., San Rafael, Ca.). The peptides were cleaved from the resin with HF and then extracted with ether and acetic acid before lyophilization. Subsequently, the peptides were purified by HPLC with the use of a reverse-phase support medium (Delta-Bondapak) on a 0.78×30 cm column with a 0-66% linear gradient of acetonitrile in 0.1% TFA.
example 2
Animals Used in Studies
[0104]Studies were performed in the transgenic (Tg) JNPL3 P301L mouse model that develops neurofibrillary tangles in several brain regions and spinal cord (Taconic, Germantown, N.Y.) (Lewis et al., “Neurofibrillary Tangles, Amyotrophy and Progressive Motor Disturbance in Mice Expressing Mutant (P301L) Tau Protein,”Nat Genet. 25:402-405 (2000), which is hereby incorporated by reference in its entirety). While this model is not ideal for AD, it is an excellent model to study the consequences of tangle development and for screening therapy that may prevent the generation of these aggregates. Another advantage of these animals is the relatively early onset of pathology. In the homozygous line, behavioral abnormalities associated with tau pathology can be observed at least as early as 3 months, but the animals remain relatively healthy at least until 8 months of age. In other words, at 8 months, the animals ambulate, feed themselves, and can perform the behavioral ...
example 3
[0107]Phos-tau peptides were mixed with Adju-Phos adjuvant (Brenntag Biosector, Denmark) at a concentration of 1 mg / ml and the solution was rotated overnight at 4° C. prior to administration to allow the peptide to adsorb onto the aluminum phosphate particles.
[0108]JNPL3 P301L mice received a subcutaneous injection of 100 μl followed by a second injection 2 weeks later and then monthly thereafter (unless otherwise indicated). Vaccination started at 2-3 months of age and continued until the animals were 8-9 months of age at which time the animals were perfused and their organs collected for analysis. The mice went through a battery of sensorimotor tests at 5-6 months and again at 8-9 months of age prior to sacrifice. Control mice received the adjuvant alone.
[0109]htau / PS1 / mtau− / − mice (n=12) were immunized with the phosphorylated tau immunogen Tau379-408[P-Ser396,404]. Three non-immunized control groups were included that received adjuvant alone. The main contro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com