Method for detecting the degree of malignancy of each of the circulating tumor cells, and a kit for the same
a technology for circulating tumor cells and kits, which is applied in the field of methods for detecting the degree of malignancy of each circulating tumor cell, and the kit for detecting the degree of malignancy of the circulating tumor cell, can solve the problems of serious wrong diagnosis, and achieve the effect of accurate diagnosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
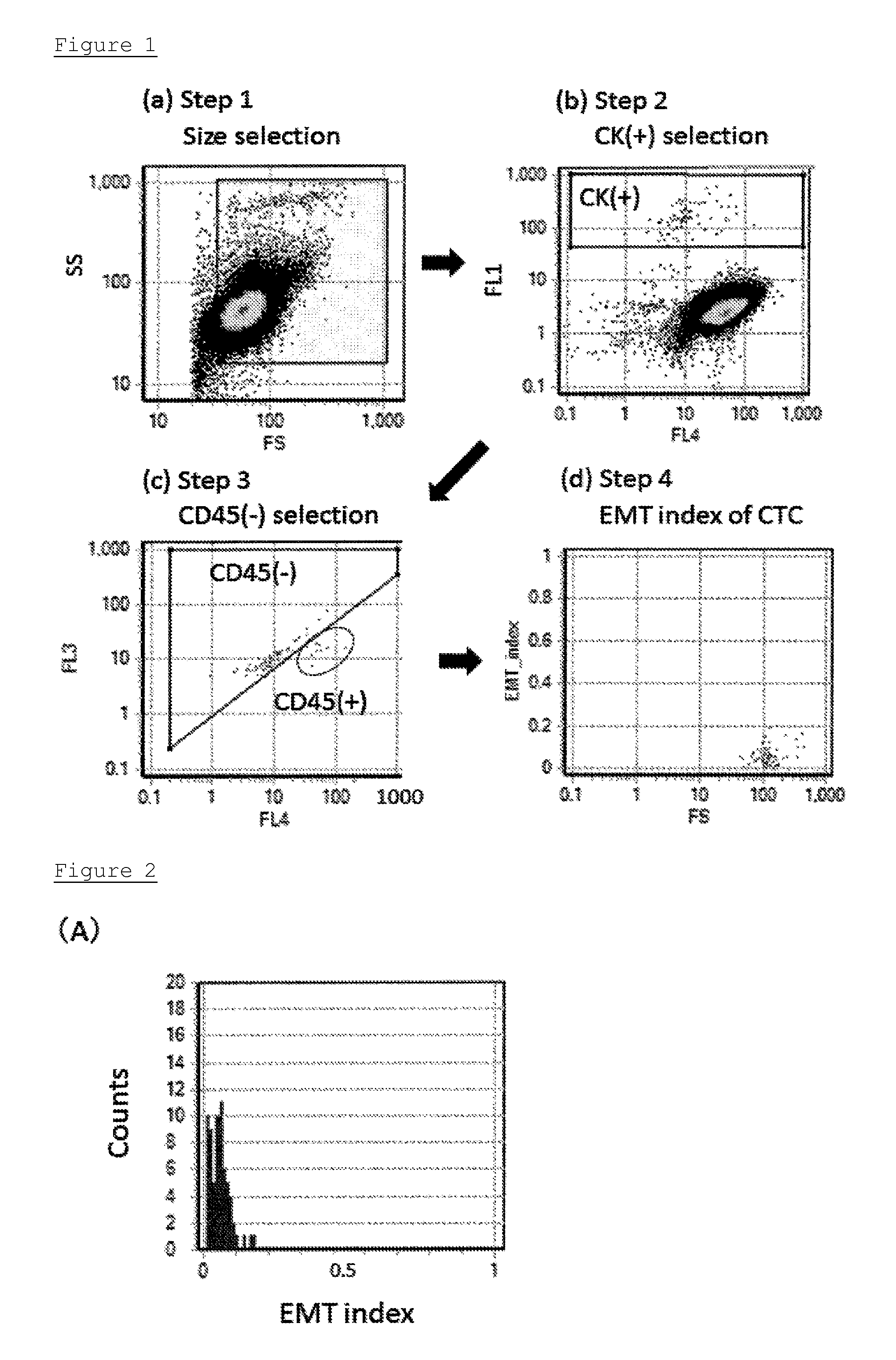
[0109]In this Example, in order to establish the detection method of the present invention, A549 cell line derived from lung cancer was mixed with the peripheral blood of the volunteer, as a substitute for CTC. Then, cytokeratin and vimentin were detected and the EMT index was calculated.
[0110]Specifically, 45 mL of lysing buffer was added to 4 mL of peripheral blood in which A549 cells (100 cells) were mixed, and the whole was mixed and allowed to stand on ice so as to lyse erythrocytes. 0.1 mL of solution wherein ImmunoTOKUI (On-chip Biotechnologies Co., Ltd) was diluted by a factor of 20 with PBS buffer containing 0.5% BSA and 2 mM EDTA (hereinafter referred to as T-buffer) was added thereto. The mixture was centrifuged, and the supernatant was aspirated so as to obtain a cell pellet. The resulting cell pellet was resuspended in 200 μL of T-buffer, and 100 μL of Fc Blocking Reagent was added thereto. The whole was incubated at 4° C. for 15 minutes. After the incubation, 200 μL of...
example 2
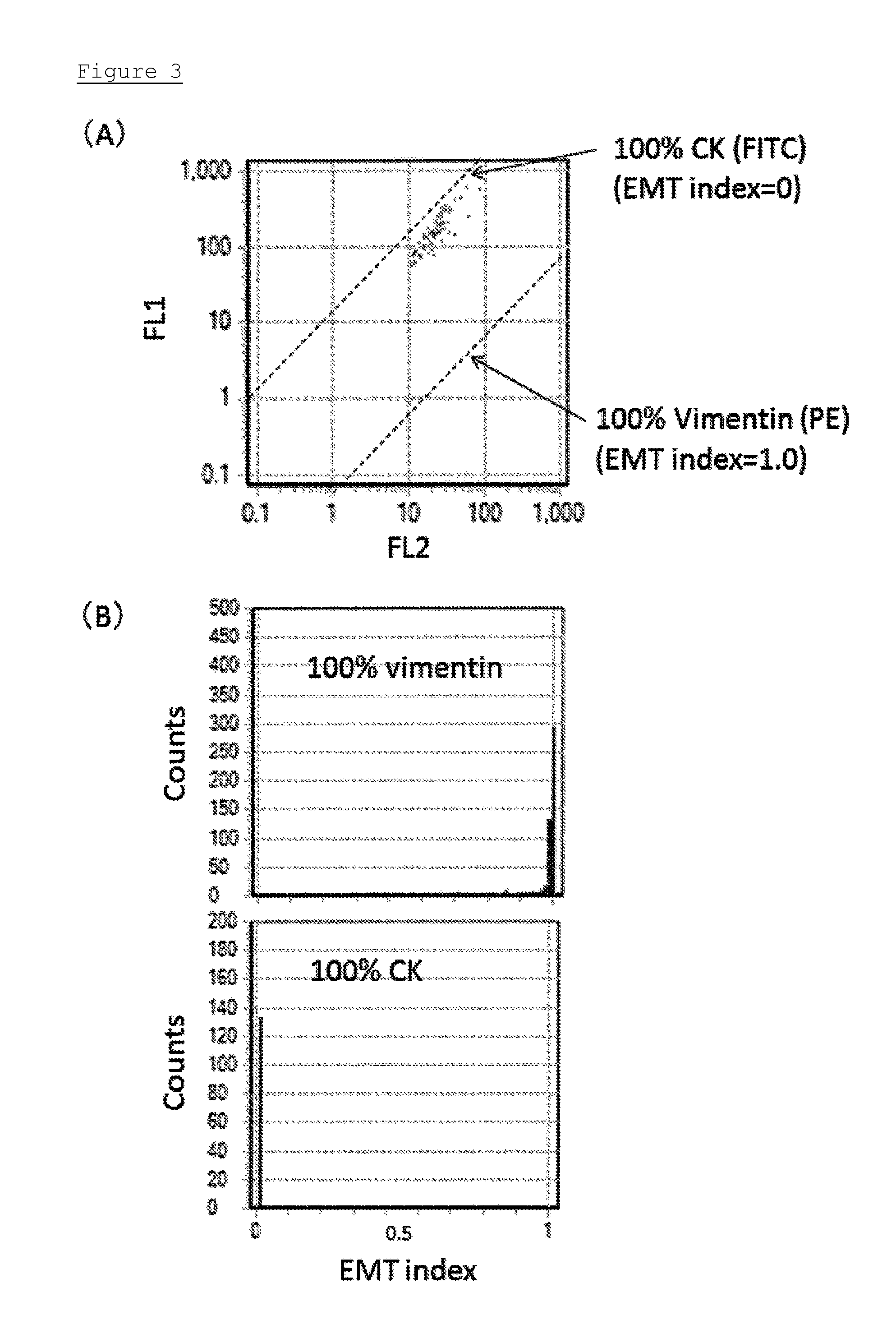
[0117]In this example, a method for determining the apparatus constants A and B of the flow cytometer in the formula i.e. EMT index=[A(FL2 / (FL1+FL2))+B] used in Example 1 is explained.
[0118]In order to obtain a standard linear of fluorescence signals of a “100% CK (FITC)” condition wherein cytokeratin only expresses and vimentin does not express, particles bound with FITC were measured by the flow cytometer. Further, in order to obtain a standard linear of fluorescence signals of a “100% Vimentin (PE)” condition wherein vimentin only expresses and cytokeratin does not express, particles bound with PE were measured by the flow cytometer.
[0119]In particular, the apparatus constants A and B are calculated as follows. In order to obtain a standard linear of fluorescence signals of a “100% CK (FITC)” condition wherein cytokeratin only expresses and vimentin does not express, the FITC-bound particles having a diameter of 3 μm were measured by the flow cytometer so as to obtain a standard ...
example 3
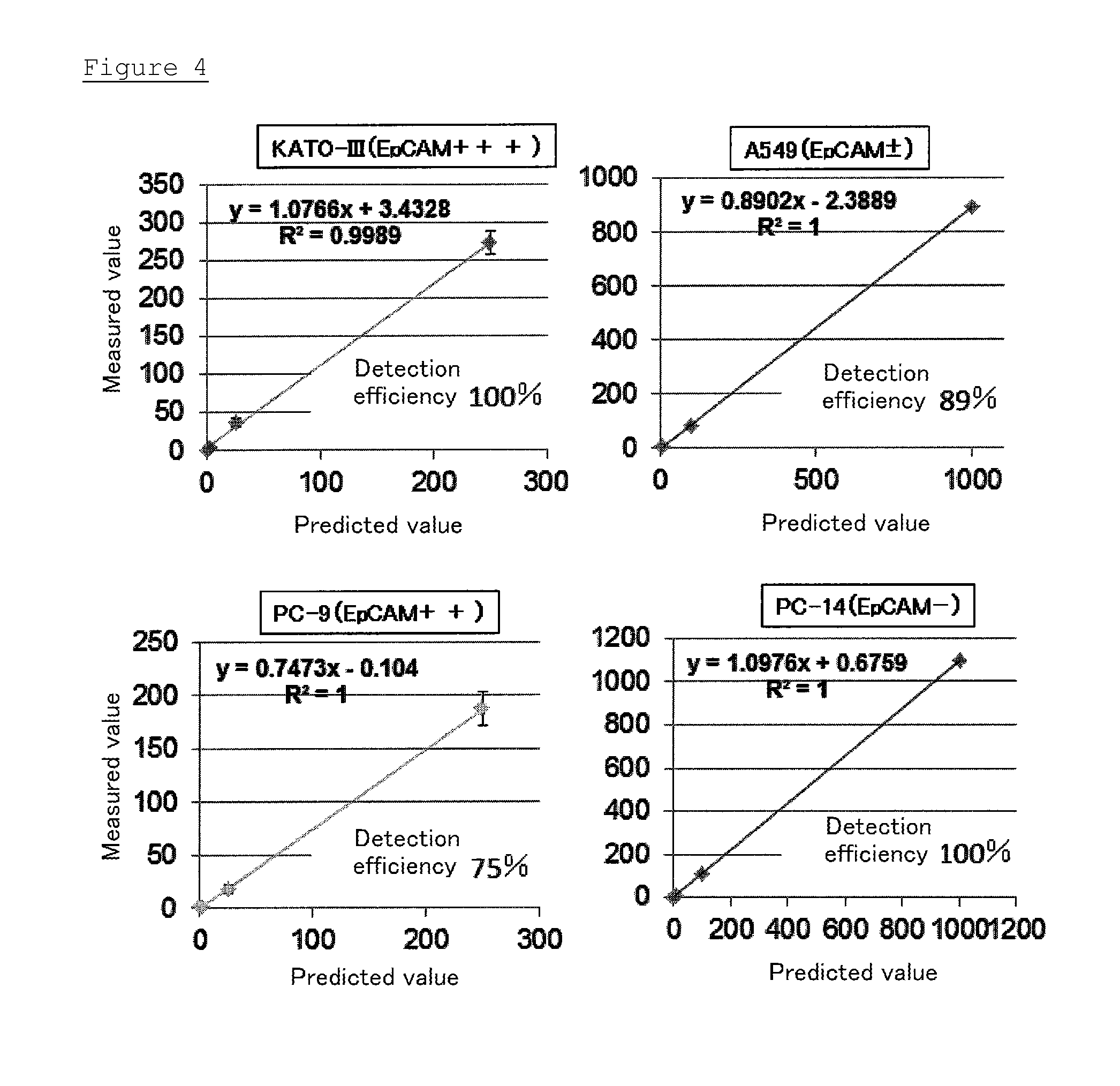
[0123]In this Example, recovery rates in the detection method of the present invention were examined by using A549 cells or cancer cells other than A549 cells.
[0124]The procedures described in Example 1 were repeated using A549 cells. Further, the procedures described in Example 1 were repeated except that KATO-III cells, PC-9 cells, or PC-14 cells were used instead of A549 cells. Then, EMT indexes thereof were calculated, and further detection rates of these cells were calculated simultaneously.
[0125]As shown in FIG. 4, the detection rates of KATO-III cells, A549 cells, PC-9 cells, and PC-14 cells were approximately 100%, 89%, 75%, and 100%, respectively.
[0126]The detection rate of PC-14 cells in which EpCAM was not expressed, was 100%. Therefore, it was revealed that the detection method of the present invention can effectively detect CTCs of metastatic cancer in which the EMT was induced.
[0127]Finally, a characteristic feature of the present invention is shown from the other poin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| fluorescence | aaaaa | aaaaa |
| luminescence | aaaaa | aaaaa |
| luminescent | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com